Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity
- PMID: 18522830
- PMCID: PMC2586840
- DOI: 10.1016/j.cmet.2008.04.002
Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity
Abstract
The polarization of adipose tissue-resident macrophages toward the alternatively activated, anti-inflammatory M2 phenotype is believed to improve insulin sensitivity. However, the mechanisms controlling tissue macrophage activation remain unclear. Here we show that adipocytes are a source of Th2 cytokines, including IL-13 and to a lesser extent IL-4, which induce macrophage PPARdelta/beta (Ppard/b) expression through a STAT6 binding site on its promoter to activate alternative activation. Coculture studies indicate that Ppard ablation renders macrophages incapable of transition to the M2 phenotype, which in turns causes inflammation and metabolic derangement in adipocytes. Remarkably, a similar regulatory mechanism by hepatocyte-derived Th2 cytokines and macrophage PPARdelta is found to control hepatic lipid metabolism. The physiological relevance of this paracrine pathway is demonstrated in myeloid-specific PPARdelta(-/-) mice, which develop insulin resistance and show increased adipocyte lipolysis and severe hepatosteatosis. These findings provide a molecular basis to modulate tissue-resident macrophage activation and insulin sensitivity.
Figures
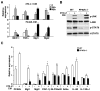


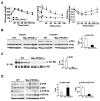

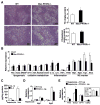
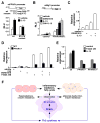
Comment in
-
PPARdelta/beta: the lobbyist switching macrophage allegiance in favor of metabolism.Cell Metab. 2008 Jun;7(6):467-9. doi: 10.1016/j.cmet.2008.05.002. Cell Metab. 2008. PMID: 18522825 Review.
References
-
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. - PubMed
-
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. - PubMed
-
- Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

