Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance
- PMID: 18522831
- PMCID: PMC2587370
- DOI: 10.1016/j.cmet.2008.04.003
Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance
Abstract
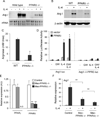
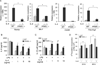
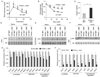
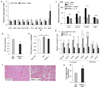
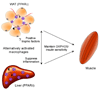
Macrophage infiltration and activation in metabolic tissues underlie obesity-induced insulin resistance and type 2 diabetes. While inflammatory activation of resident hepatic macrophages potentiates insulin resistance, the functions of alternatively activated Kupffer cells in metabolic disease remain unknown. Here we show that in response to the Th2 cytokine interleukin-4 (IL-4), peroxisome proliferator-activated receptor delta (PPARdelta) directs expression of the alternative phenotype in Kupffer cells and adipose tissue macrophages of lean mice. However, adoptive transfer of PPARdelta(-/-) (Ppard(-/-)) bone marrow into wild-type mice diminishes alternative activation of hepatic macrophages, causing hepatic dysfunction and systemic insulin resistance. Suppression of hepatic oxidative metabolism is recapitulated by treatment of primary hepatocytes with conditioned medium from PPARdelta(-/-) macrophages, indicating direct involvement of Kupffer cells in liver lipid metabolism. Taken together, these data suggest an unexpected beneficial role for alternatively activated Kupffer cells in metabolic syndrome and type 2 diabetes.
Figures


Comment in
-
Kupffer cells and hepatocyte metabolism: a two-way street?Hepatology. 2009 Feb;49(2):690-2. doi: 10.1002/hep.22801. Hepatology. 2009. PMID: 19177564 No abstract available.
References
-
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. - PubMed
-
- Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

