Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice
- PMID: 18522833
- PMCID: PMC2474721
- DOI: 10.1016/j.cmet.2008.04.011
Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice
Abstract
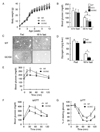
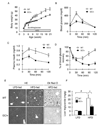
The molecular mechanisms linking the stress of unfolded proteins in the endoplasmic reticulum (ER stress) to glucose intolerance in obese animals are poorly understood. In this study, enforced expression of a translation initiation factor 2alpha (eIF2alpha)-specific phosphatase, GADD34, was used to selectively compromise signaling in the eIF2(alphaP)-dependent arm of the ER unfolded protein response in liver of transgenic mice. The transgene resulted in lower liver glycogen levels and susceptibility to fasting hypoglycemia in lean mice and glucose tolerance and diminished hepatosteatosis in animals fed a high-fat diet. Attenuated eIF2(alphaP) correlated with lower expression of the adipogenic nuclear receptor PPARgamma and its upstream regulators, the transcription factors C/EBPalpha and C/EBPbeta, in transgenic mouse liver, whereas eIF2alpha phosphorylation promoted C/EBP translation in cultured cells and primary hepatocytes. These observations suggest that eIF2(alphaP)-mediated translation of key hepatic transcriptional regulators of intermediary metabolism contributes to the detrimental consequences of nutrient excess.
Figures




References
-
- Allen-Jennings AE, Hartman MG, Kociba GJ, Hai T. The roles of ATF3 in liver dysfunction and the regulation of phosphoenolpyruvate carboxykinase gene expression. J Biol Chem. 2002;277:20020–20025. - PubMed
-
- Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. - PubMed
-
- Chen C, Dudenhausen EE, Pan YX, Zhong C, Kilberg MS. Human CCAAT/enhancer-binding protein beta gene expression is activated by endoplasmic reticulum stress through an unfolded protein response element downstream of the protein coding sequence. J Biol Chem. 2004;279:27948–27956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

