The prevalence of folate-remedial MTHFR enzyme variants in humans
- PMID: 18523009
- PMCID: PMC2430358
- DOI: 10.1073/pnas.0802813105
The prevalence of folate-remedial MTHFR enzyme variants in humans
Abstract
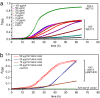
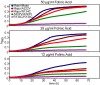
Studies of rare, inborn metabolic diseases establish that the phenotypes of some mutations in vitamin-dependent enzymes can be suppressed by supplementation of the cognate vitamin, which restores function of the defective enzyme. To determine whether polymorphisms exist that more subtly affect enzymes yet are augmentable in the same way, we sequenced the coding region of a prototypical vitamin-dependent enzyme, methylenetetrahydrofolate reductase (MTHFR), from 564 individuals of diverse ethnicities. All nonsynonymous changes were evaluated in functional in vivo assays in Saccharomyces cerevisiae to identify enzymatic defects and folate remediability of impaired alleles. We identified 14 nonsynonymous changes: 11 alleles with minor allele frequencies <1% and 3 common alleles (A222V, E429A, and R594Q). Four of 11 low-frequency alleles affected enzyme function, as did A222V. Of the five impaired alleles, four could be restored to normal functionality by elevating intracellular folate levels. All five impaired alleles mapped to the N-terminal catalytic domain of the enzyme, whereas changes in the C-terminal regulatory domain had little effect on activity. Impaired activity correlated with the phosphorylation state of MTHFR, with more severe mutations resulting in lower abundance of the phosphorylated protein. Significantly, diploid yeast heterozygous for mutant alleles were impaired for growth, particularly with lower folate supplementation. These results suggested that multiple less-frequent alleles, in aggregate, might significantly contribute to metabolic dysfunction. Furthermore, vitamin remediation of mutant enzymes may be a common phenomenon in certain domains of proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Scriver CR. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw–Hill; 2001.
-
- Ames BN, Elson-Schwab I, Silver EA. High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased k(m)): Relevance to genetic disease and polymorphisms. Am J Clin Nutr. 2002;75:616–658. - PubMed
-
- Clayton PT. B6-responsive disorders: A model of vitamin dependency. J Inherit Metab Dis. 2006;29:317–326. - PubMed
-
- Perez B, et al. Kinetic and stability analysis of pku mutations identified in bh4-responsive patients. Mol Genet Metab. 2005;86(Suppl 1):S11–S16. - PubMed
-
- Wittung-Stafshede P. Role of cofactors in protein folding. Acc Chem Res. 2002;35:201–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

