Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex
- PMID: 18523013
- PMCID: PMC2430372
- DOI: 10.1073/pnas.0803849105
Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex
Abstract
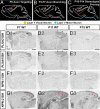
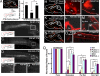
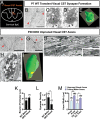
Neurons in the developing CNS tend to send out long axon collaterals to multiple target areas. For these neurons to attain specific connections, some of their axon collaterals are subsequently pruned-a process called stereotyped axon pruning. One of the most striking examples of stereotyped pruning in the CNS is the pruning of corticospinal tract (CST) axons. The long CST collaterals from layer V neurons of the visual and motor cortices are differentially pruned during development. Here we demonstrate that select plexins and neuropilins, which serve as coreceptors for semaphorins, are expressed in visual cortical neurons at the time when CST axon collaterals are stereotypically pruned. By analyzing mutant mice, we find that the pruning of visual, but not motor, CST axon collaterals depends on plexin-A3, plexin-A4, and neuropilin-2. Expression pattern study suggests that Sema3F is a candidate local cue for the pruning of visual CST axons. Using electron microscopic analysis, we also show that visual CST axon collaterals form synaptic contacts in the spinal cord before pruning and that the unpruned collaterals in adult mutant mice are unmyelinated and maintain their synaptic contacts. Our results indicate that the stereotyped pruning of the visual and motor CST axon collaterals is differentially regulated and that this specificity arises from the differential expression of plexin receptors in the cortex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Dorsal turning of motor corticospinal axons at the pyramidal decussation requires plexin signaling.Neural Dev. 2008 Aug 26;3:21. doi: 10.1186/1749-8104-3-21. Neural Dev. 2008. PMID: 18727829 Free PMC article.
-
Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus.J Neurosci. 2005 Oct 5;25(40):9124-34. doi: 10.1523/JNEUROSCI.2648-05.2005. J Neurosci. 2005. PMID: 16207871 Free PMC article.
-
Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family.Cell. 2003 May 2;113(3):285-99. doi: 10.1016/s0092-8674(03)00267-8. Cell. 2003. PMID: 12732138
-
Axon pruning and synaptic development: how are they per-plexin?Neuroscientist. 2006 Oct;12(5):398-409. doi: 10.1177/1073858406292631. Neuroscientist. 2006. PMID: 16957002 Review.
-
Axon pruning in the developing vertebrate hippocampus.Dev Neurosci. 2007;29(1-2):6-13. doi: 10.1159/000096207. Dev Neurosci. 2007. PMID: 17148945 Review.
Cited by
-
An increase in Semaphorin 3A biases the axonal direction and induces an aberrant dendritic arborization in an in vitro model of human neural progenitor differentiation.Cell Biosci. 2022 Nov 8;12(1):182. doi: 10.1186/s13578-022-00916-1. Cell Biosci. 2022. PMID: 36348448 Free PMC article.
-
Targeted sequencing and functional analysis reveal brain-size-related genes and their networks in autism spectrum disorders.Mol Psychiatry. 2017 Sep;22(9):1282-1290. doi: 10.1038/mp.2017.140. Epub 2017 Jul 25. Mol Psychiatry. 2017. PMID: 28831199
-
Genetics and cell biology of building specific synaptic connectivity.Annu Rev Neurosci. 2010;33:473-507. doi: 10.1146/annurev.neuro.051508.135302. Annu Rev Neurosci. 2010. PMID: 20367446 Free PMC article. Review.
-
Semaphorins and the dynamic regulation of synapse assembly, refinement, and function.Curr Opin Neurobiol. 2014 Aug;27:1-7. doi: 10.1016/j.conb.2014.02.005. Epub 2014 Mar 2. Curr Opin Neurobiol. 2014. PMID: 24598309 Free PMC article. Review.
-
Sculpting neural circuits by axon and dendrite pruning.Annu Rev Cell Dev Biol. 2015;31:779-805. doi: 10.1146/annurev-cellbio-100913-013038. Epub 2015 Oct 2. Annu Rev Cell Dev Biol. 2015. PMID: 26436703 Free PMC article. Review.
References
-
- Kantor DB, Kolodkin AL. Curbing the excesses of youth: Molecular insights into axonal pruning. Neuron. 2003;38:849–852. - PubMed
-
- Low LK, Cheng HJ. A little nip and tuck: Axon refinement during development and axonal injury. Curr Opin Neurobiol. 2005;15:549–556. - PubMed
-
- Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. - PubMed
-
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6:955–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

