The Tec family kinase, IL-2-inducible T cell kinase, differentially controls mast cell responses
- PMID: 18523250
- PMCID: PMC2583454
- DOI: 10.4049/jimmunol.180.12.7869
The Tec family kinase, IL-2-inducible T cell kinase, differentially controls mast cell responses
Abstract
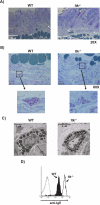
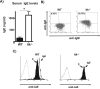
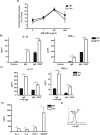
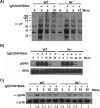
The Tec family tyrosine kinase, IL-2-inducible T cell kinase (Itk), is expressed in T cells and mast cells. Mice lacking Itk exhibit impaired Th2 cytokine secretion; however, they have increased circulating serum IgE, but exhibit few immunological symptoms of allergic airway responses. We have examined the role of Itk in mast cell function and FcepsilonRI signaling. We report in this study that Itk null mice have reduced allergen/IgE-induced histamine release, as well as early airway hyperresponsiveness in vivo. This is due to the increased levels of IgE in the serum of these mice, because the transfer of Itk null bone marrow-derived cultured mast cells into mast cell-deficient W/W(v) animals is able to fully rescue histamine release in the W/W(v) mice. Further analysis of Itk null bone marrow-derived cultured mast cells in vitro revealed that whereas they have normal degranulation responses, they secrete elevated levels of cytokines, including IL-13 and TNF-alpha, particularly in response to unliganded IgE. Analysis of biochemical events downstream of the FcepsilonRI revealed little difference in overall tyrosine phosphorylation of specific substrates or calcium responses; however, these cells express elevated levels of NFAT, which was largely nuclear. Our results suggest that the reduced mast cell response in vivo in Itk null mice is due to elevated levels of IgE in these mice. Our results also suggest that Itk differentially modulates mast cell degranulation and cytokine production in part by regulating expression and activation of NFAT proteins in these cells.
Figures




References
-
- Marone G, Triggiani M, de Paulis A. Mast cells and basophils: friends as well as foes in bronchial asthma? Trends Immunol. 2005;26:25–31. - PubMed
-
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. - PubMed
-
- Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. - PubMed
-
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. - PubMed
-
- Sayed BA, Brown MA. Mast cells as modulators of T-cell responses. Immunol Rev. 2007;217:53–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

