Misoprostol impairs female reproductive tract innate immunity against Clostridium sordellii
- PMID: 18523288
- PMCID: PMC2667109
- DOI: 10.4049/jimmunol.180.12.8222
Misoprostol impairs female reproductive tract innate immunity against Clostridium sordellii
Abstract
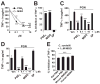
Fatal cases of acute shock complicating Clostridium sordellii endometritis following medical abortion with mifepristone (also known as RU-486) used with misoprostol were reported. The pathogenesis of this unexpected complication remains enigmatic. Misoprostol is a pharmacomimetic of PGE(2), an endogenous suppressor of innate immunity. Clinical C. sordellii infections were associated with intravaginal misoprostol administration, suggesting that high misoprostol concentrations within the uterus impair immune responses against C. sordellii. We modeled C. sordellii endometritis in rats to test this hypothesis. The intrauterine but not the intragastric delivery of misoprostol significantly worsened mortality from C. sordellii uterine infection, and impaired bacterial clearance in vivo. Misoprostol also reduced TNF-alpha production within the uterus during infection. The intrauterine injection of misoprostol did not enhance mortality from infection by the vaginal commensal bacterium Lactobacillus crispatus. In vitro, misoprostol suppressed macrophage TNF-alpha and chemokine generation following C. sordellii or peptidoglycan challenge, impaired leukocyte phagocytosis of C. sordellii, and inhibited uterine epithelial cell human beta-defensin expression. These immunosuppressive effects of misoprostol, which were not shared by mifepristone, correlated with the activation of the G(s) protein-coupled E prostanoid (EP) receptors EP2 and EP4 (macrophages) or EP4 alone (uterine epithelial cells). Our data provide a novel explanation for postabortion sepsis leading to death and also suggest that PGE(2), in which production is exaggerated within the reproductive tract during pregnancy, might be an important causal determinant in the pathogenesis of more common infections of the gravid uterus.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures
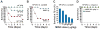
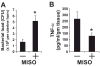
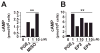
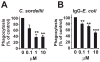
Comment in
-
Comment on "Misoprostol impairs female reproductive tract innate immunity against Clostridium sordellii".J Immunol. 2008 Aug 15;181(4):2263; author reply 2263-4. doi: 10.4049/jimmunol.181.4.2263. J Immunol. 2008. PMID: 18684913 No abstract available.
References
-
- Fischer M, Bhatnagar J, Guarner J, Reagan S, Hacker JK, Van Meter SH, Poukens V, Whiteman DB, Iton A, Cheung M, et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N Engl J Med. 2005;353:2352–2360. - PubMed
-
- Sinave C, Le Templier G, Blouin D, Leveille F, Deland E. Toxic shock syndrome due to Clostridium sordellii: a dramatic postpartum and post-abortion disease. Clin Infect Dis. 2002;35:1441–1443. - PubMed
-
- Cohen AL, Bhatnagar J, Reagan S, Zane SB, D’Angeli MA, Fischer M, Killgore G, Kwan-Gett TS, Blossom DB, Shieh WJ, et al. Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstet Gynecol. 2007;110:1027–1033. - PubMed
-
- Miech RP. Pathophysiology of mifepristone-induced septic shock due to Clostridium sordellii. Ann Pharmacother. 2005;39:1483–1488. - PubMed
-
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

