The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine following peroral administration route
- PMID: 18523770
- PMCID: PMC11030077
- DOI: 10.1007/s00262-008-0539-9
The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine following peroral administration route
Abstract
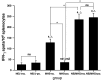
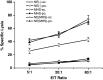
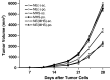
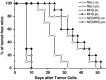
Previous studies have shown that there are profuse lymphatic tissues under the intestinal mucous membrane. Moreover, vaccine administered orally can elicit both mucous membrane and system immune response simultaneously, accordingly induce tumor-specific cytotoxic T lymphocyte. As a result, the oral route is constituted the preferred immune route for vaccine delivery theoretically. However, numerous vaccines especially protein/peptide vaccines remain poorly available when administered by this route. Nanoemulsion has been shown as a useful vehicle can be developed to enhance the antitumor immune response against antigens encapsulated in it and it is good for the different administration routes. Of particular interest is whether the protein vaccine following peroral route using nanoemulsion as delivery carrier can induce the same, so much as stronger antitumor immune response to following conventional ways such as subcutaneous (sc.) or not. Hence, in the present study, we encapsulated the MAGE1-HSP70 and SEA complex protein in nanoemulsion as nanovaccine NE (MHS) using magnetic ultrasound method. We then immuned C57BL/6 mice with NE (MHS), MHS alone or NE (-) via po. or sc. route and detected the cellular immunocompetence by using ELISpot assay and LDH release assay. The therapeutic and tumor challenge assay were examined then. The results showed that compared with vaccination with MHS or NE (-), the cellular immune responses against MAGE-1 could be elicited fiercely by vaccination with NE (MHS) nanoemulsion. Furthermore, encapsulating MHS in nanoemulsion could delay tumor growth and defer tumor occurrence of mice challenged with B16-MAGE-1 tumor cells. Especially, the peroral administration of NE (MHS) could induce approximately similar antitumor immune responses to the sc. administration, but the MHS unencapsulated with nanoemulsion via po. could induce significantly weaker antitumor immune responses than that via sc., suggesting nanoemulsion as a promising carrier can exert potent antitumor immunity against antigen encapsulated in it and make the tumor protein vaccine immunizing via po. route feasible and effective. It may have a broad application in tumor protein vaccine.
Figures

Similar articles
-
The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine following different administration routes.Oncol Rep. 2009 Oct;22(4):915-20. doi: 10.3892/or_00000517. Oncol Rep. 2009. PMID: 19724873
-
Tomato lectin-modified nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine: Targeting intestinal M cells following peroral administration.Biomed Pharmacother. 2019 Jul;115:108886. doi: 10.1016/j.biopha.2019.108886. Epub 2019 Apr 28. Biomed Pharmacother. 2019. PMID: 31029887
-
Preparation of a new combination nanoemulsion-encapsulated MAGE1-MAGE3-MAGEn/HSP70 vaccine and study of its immunotherapeutic effect.Pathol Res Pract. 2020 Jun;216(6):152954. doi: 10.1016/j.prp.2020.152954. Epub 2020 Apr 17. Pathol Res Pract. 2020. PMID: 32321658
-
Advancements in Nanoemulsion-Based Drug Delivery Across Different Administration Routes.Pharmaceutics. 2025 Mar 5;17(3):337. doi: 10.3390/pharmaceutics17030337. Pharmaceutics. 2025. PMID: 40143001 Free PMC article. Review.
-
Recent Developments in Oral Delivery of Vaccines Using Nanocarriers.Vaccines (Basel). 2023 Feb 20;11(2):490. doi: 10.3390/vaccines11020490. Vaccines (Basel). 2023. PMID: 36851367 Free PMC article. Review.
Cited by
-
Nanoparticle drug delivery systems: an excellent carrier for tumor peptide vaccines.Drug Deliv. 2018 Nov;25(1):1319-1327. doi: 10.1080/10717544.2018.1477857. Drug Deliv. 2018. PMID: 29869539 Free PMC article. Review.
-
Gas‑filled ultrasound microbubbles enhance the immunoactivity of the HSP70‑MAGEA1 fusion protein against MAGEA1‑expressing tumours.Mol Med Rep. 2018 Jul;18(1):315-321. doi: 10.3892/mmr.2018.9003. Epub 2018 May 9. Mol Med Rep. 2018. PMID: 29749485 Free PMC article.
-
Protein-based nanoparticles in cancer vaccine development.Nanomedicine. 2019 Jan;15(1):164-174. doi: 10.1016/j.nano.2018.09.004. Epub 2018 Oct 4. Nanomedicine. 2019. PMID: 30291897 Free PMC article. Review.
-
Diversity of extracellular HSP70 in cancer: advancing from a molecular biomarker to a novel therapeutic target.Front Oncol. 2024 Apr 5;14:1388999. doi: 10.3389/fonc.2024.1388999. eCollection 2024. Front Oncol. 2024. PMID: 38646439 Free PMC article. Review.
-
Emerging vaccine nanotechnology: From defense against infection to sniping cancer.Acta Pharm Sin B. 2022 May;12(5):2206-2223. doi: 10.1016/j.apsb.2021.12.021. Epub 2022 Jan 4. Acta Pharm Sin B. 2022. PMID: 35013704 Free PMC article. Review.
References
-
- Alpar HO, Field WN, Hayes K, Lewis DA. A possible use of orally administered microspheres in the treatment of inflammation. J Pharm Pharmacol. 1989;41(Suppl):50. - PubMed
-
- Ammoury N, Fessi H, Devissaguet JP, Dubrasquet M, Benita S. Jejunal absorption, pharmacological activity, and pharmacokinetic evaluation of indomethacin-loaded poly(d,l-lactide) and poly(isobutyl-cyanoacrylate) nanocapsules in rats. Pharm Res. 1991;8:101. doi: 10.1023/A:1015846810474. - DOI - PubMed
-
- Beck PH, Kreuter J, Müller WEG, Schatton W. Improved peroral delivery of avarol with polyalkylcyanoacrylate nanoparticles. Eur J Pharm Biopharm. 1994;40:134.
-
- Bonduelle S, Carrier M, Pimienta C, Benoit JP, Lenaerts V. Tissue concentration of nanoencapsulated radiolabelled cyclosporin following peroral delivery in mice or ophthalmic applications in rabbits. Eur J Pharm Biopharm. 1996;42:313.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

