Genetic regulation of arealization of the neocortex
- PMID: 18524571
- PMCID: PMC2677555
- DOI: 10.1016/j.conb.2008.05.011
Genetic regulation of arealization of the neocortex
Abstract
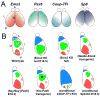
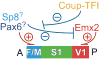
Arealization of the neocortex is controlled by a regulatory hierarchy beginning with morphogens secreted from patterning centers positioned at the perimeter of the dorsal telencephalon. These morphogens act in part to establish within cortical progenitors the differential expression of transcription factors that specify their area identity, which is inherited by their neuronal progeny, providing the genetic framework for area patterning. The two patterning centers most directly implicated in arealization are the commissural plate, which expresses fibroblast growth factors, and the cortical hem, which expresses bone morphogenetic proteins and vertebrate orthologs of Drosophila wingless, the Wnts. A third, albeit putative, patterning center is the antihem, identified by its expression of multiple signaling molecules. We describe recent findings on roles for these patterning centers in arealization. We also present the most recent evidence on functions of the four transcription factors, Emx2, COUP-TFI, Pax6, and Sp8, thus far implicated in arealization. We also describe screens for candidate target genes of these transcription factors, or other genes potentially involved in arealization. We conclude with an assessment of a forward genetics approach for identifying genes involved in determining area size based in part on quantitative trait locus mapping, and the implications for significant differences between individuals in area size on behavioral performance.
Figures


References
-
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. - PubMed
-
- O’Leary DD. Do cortical areas emerge from a protocortex? Trends Neurosci. 1989;12:400–406. - PubMed
-
- O’Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. - PubMed
-
- Rash BG, Grove EA. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 2006;16:25–34. - PubMed
-
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

