Effects of lamin A/C, lamin B1, and viral US3 kinase activity on viral infectivity, virion egress, and the targeting of herpes simplex virus U(L)34-encoded protein to the inner nuclear membrane
- PMID: 18524819
- PMCID: PMC2519571
- DOI: 10.1128/JVI.00874-08
Effects of lamin A/C, lamin B1, and viral US3 kinase activity on viral infectivity, virion egress, and the targeting of herpes simplex virus U(L)34-encoded protein to the inner nuclear membrane
Abstract
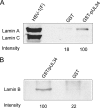
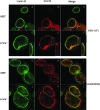
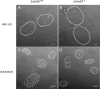
Previous results indicated that the U(L)34 protein (pU(L)34) of herpes simplex virus 1 (HSV-1) is targeted to the nuclear membrane and is essential for nuclear egress of nucleocapsids. The normal localization of pU(L)34 and virions requires the U(S)3-encoded kinase that phosphorylates U(L)34 and lamin A/C. Moreover, pU(L)34 was shown to interact with lamin A in vitro. In the present study, glutathione S-transferase/pU(L)34 was shown to specifically pull down lamin A and lamin B1 from cellular lysates. To determine the role of these interactions on viral infectivity and pU(L)34 targeting to the inner nuclear membrane (INM), the localization of pU(L)34 was determined in LmnA(-/-) and LmnB1(-/-) mouse embryonic fibroblasts (MEFs) by indirect immunofluorescence and immunogold electron microscopy in the presence or absence of U(S)3 kinase activity. While pU(L)34 INM targeting was not affected by the absence of lamin B1 in MEFs infected with wild-type HSV as viewed by indirect immunofluorescence, it localized in densely staining scalloped-shaped distortions of the nuclear membrane in lamin B1 knockout cells infected with a U(S)3 kinase-dead virus. Lamin B1 knockout cells were relatively less permissive for viral replication than wild-type MEFs, with viral titers decreased at least 10-fold. The absence of lamin A (i) caused clustering of pU(L)34 in the nuclear rim of cells infected with wild-type virus, (ii) produced extensions of the INM bearing pU(L)34 protein in cells infected with a U(S)3 kinase-dead mutant, (iii) precluded accumulation of virions in the perinuclear space of cells infected with this mutant, and (iv) partially restored replication of this virus. The latter observation suggests that lamin A normally impedes viral infectivity and that U(S)3 kinase activity partially alleviates this impediment. On the other hand, lamin B1 is necessary for optimal viral replication, probably through its well-documented effects on many cellular pathways. Finally, neither lamin A nor B1 was absolutely required for targeting pU(L)34 to the INM, suggesting that this targeting is mediated by redundant functions or can be mediated by other proteins.
Figures




References
-
- Baines, J. D., and C. Duffy. 2006. Nucleocapsid assembly and envelopment of herpes simplex virus, p. 175-204. In R. M. Sandri-Goldin (ed.), Alpha herpesviruses: pathogenesis, molecular biology and infection control. Caister Scientific Press, Norfolk, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

