Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2
- PMID: 18524855
- PMCID: PMC2519178
- DOI: 10.1152/ajprenal.90300.2008
Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2
Abstract
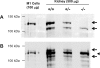
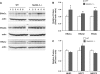
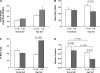
Nedd4-2 has been proposed to play a critical role in regulating epithelial Na+ channel (ENaC) activity. Biochemical and overexpression experiments suggest that Nedd4-2 binds to the PY motifs of ENaC subunits via its WW domains, ubiquitinates them, and decreases their expression on the apical membrane. Phosphorylation of Nedd4-2 (for example by Sgk1) may regulate its binding to ENaC, and thus ENaC ubiquitination. These results suggest that the interaction between Nedd4-2 and ENaC may play a crucial role in Na+ homeostasis and blood pressure (BP) regulation. To test these predictions in vivo, we generated Nedd4-2 null mice. The knockout mice had higher BP on a normal diet and a further increase in BP when on a high-salt diet. The hypertension was probably mediated by ENaC overactivity because 1) Nedd4-2 null mice had higher expression levels of all three ENaC subunits in kidney, but not of other Na+ transporters; 2) the downregulation of ENaC function in colon was impaired; and 3) NaCl-sensitive hypertension was substantially reduced in the presence of amiloride, a specific inhibitor of ENaC. Nedd4-2 null mice on a chronic high-salt diet showed cardiac hypertrophy and markedly depressed cardiac function. Overall, our results demonstrate that in vivo Nedd4-2 is a critical regulator of ENaC activity and BP. The absence of this gene is sufficient to produce salt-sensitive hypertension. This model provides an opportunity to further investigate mechanisms and consequences of this common disorder.
Figures

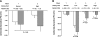




References
-
- Abriel H, Staub O. Ubiquitylation of ion channels. Physiology Bethesda 20: 398–407, 2005. - PubMed
-
- Carlson SH, Wyss JM. Long-term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension 35: E1–E5, 2000. - PubMed
-
- Chen H, Ross CA, Wang N, Huo Y, MacKinnon DF, Potash JB, Simpson SG, McMahon FJ, DePaulo JR Jr, McInnis MG. NEDD4L on human chromosome 18q21 has multiple forms of transcripts and is a homologue of the mouse Nedd4-2 gene. Eur J Hum Genet 9: 922–930, 2001. - PubMed
-
- Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O'Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 46: 156–161, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

