Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor
- PMID: 18524885
- PMCID: PMC3766527
- DOI: 10.1124/mol.108.048884
Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor
Abstract
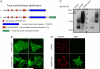
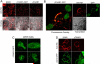
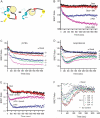
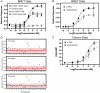
Trace amines are neurotransmitters whose role in regulating invertebrate physiology has been appreciated for many decades. Recent studies indicate that trace amines may also play a role in mammalian physiology by binding to a novel family of G protein-coupled receptors (GPCRs) that are found throughout the central nervous system. A major obstacle impeding the careful pharmacological characterization of trace amine associated receptors (TAARs) is their extremely poor membrane expression in model cell systems, and a molecular basis for this phenomenon has not been determined. In the present study, we show that the addition of an asparagine-linked glycosylation site to the N terminus of the human trace amine associated receptor 1 (TAAR1) is sufficient to enable its plasma membrane expression, and thus its pharmacological characterization with a novel cAMP EPAC (exchange protein directly activated by cAMP) protein based bioluminescence resonance energy transfer (BRET) biosensor. We applied this novel cAMP BRET biosensor to evaluate the activity of putative TAAR1 ligands. This study represents the first comprehensive investigation of the membrane-expressed human TAAR1 pharmacology. Our strategy to express TAARs and to identify their ligands using a cAMP BRET assay could provide a foundation for characterizing the functional role of trace amines in vivo and suggests a strategy to apply to groups of poorly expressing GPCRs that have remained difficult to investigate in model systems.
Figures
References
-
- Barak LS, Ferguson SS, Zhang J, Caron MG. A β-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem. 1997;272:27497–27500. - PubMed
-
- Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem. 2004;90:257–271. - PubMed
-
- Boulton AA. Trace amines and mental disorders. Can J Neurol Sci. 1980;7:261–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

