Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege
- PMID: 18524901
- PMCID: PMC6670327
- DOI: 10.1523/JNEUROSCI.0060-08.2008
Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege
Abstract
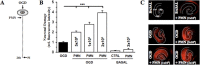
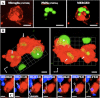
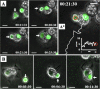
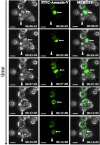
Microglial cells maintain the immunological integrity of the healthy brain and can exert protection from traumatic injury. During ischemic tissue damage such as stroke, peripheral immune cells acutely infiltrate the brain and may exacerbate neurodegeneration. Whether and how microglia can protect from this insult is unknown. Polymorphonuclear neutrophils (PMNs) are a prominent immunologic infiltrate of ischemic lesions in vivo. Here, we show in organotypic brain slices that externally applied invading PMNs massively enhance ischemic neurotoxicity. This, however, is counteracted by additional application of microglia. Time-lapse imaging shows that microglia exert protection by rapid engulfment of apoptotic, but, strikingly, also viable, motile PMNs in cell culture and within brain slices. PMN engulfment is mediated by integrin- and lectin-based recognition. Interference with this process using RGDS peptides and N-acetyl-glucosamine blocks engulfment of PMNs and completely abrogates the neuroprotective function of microglia. Thus, engulfment of invading PMNs by microglia may represent an entirely new mechanism of CNS immune privilege.
Figures



References
-
- Arai H, Furuya T, Yasuda T, Miura M, Mizuno Y, Mochizuki H. Neurotoxic effects of lipopolysaccharide on nigral dopaminergic neurons are mediated by microglial activation, interleukin-1beta, and expression of caspase-11 in mice. J Biol Chem. 2004;279:51647–51653. - PubMed
-
- Arumugam TV, Granger DN, Mattson MP. Stroke and T-cells. Neuromol Med. 2005;7:229–242. - PubMed
-
- Baldauf K, Reymann KG. Influence of EGF/bFGF treatment on proliferation, early neurogenesis and infarct volume after transient focal ischemia. Brain Res. 2005;1056:158–167. - PubMed
-
- Banati RB, Graeber MB. Surveillance, intervention and cytotoxicity: is there a protective role of microglia? Dev Neurosci. 1994;16:114–127. - PubMed
-
- Barone FC, Hillegass LM, Price WJ, White RF, Lee EV, Feuerstein GZ, Sarau HM, Clark RK, Griswold DE. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res. 1991;29:336–345. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
