Gustatory neural circuitry in the hamster brain stem
- PMID: 18525019
- PMCID: PMC2525717
- DOI: 10.1152/jn.01364.2007
Gustatory neural circuitry in the hamster brain stem
Abstract
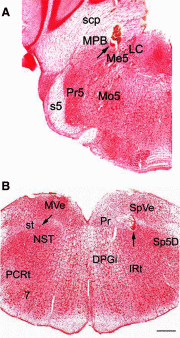
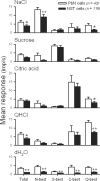
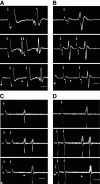
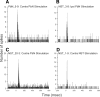
The nucleus of the solitary tract (NST) and the parabrachial nuclei (PbN) are the first and second central relays for the taste pathway, respectively. Taste neurons in the NST project to the PbN, which further transmits taste information to the rostral taste centers. Nevertheless, details of the neural connections among the brain stem gustatory nuclei are obscure. Here, we investigated these relationships in the hamster brain stem. Three electrode assemblies were used to record the activity of taste neurons extracellularly and then to electrically stimulate these same areas in the order: left PbN, right PbN, and right NST. A fourth electrode, a glass micropipette, was used to record from gustatory cells in the left NST. Results showed extensive bilateral communication between brain stem nuclei at the same level: 1) 10% of 96 NST neurons projected to the contralateral NST and 58% received synaptic input from the contralateral NST; and 2) 12% of 43 PbN neurons projected to the contralateral PbN and 21% received synaptic input from the contralateral PbN. Results also showed extensive communication between levels: 1) as expected, the majority of 119 NST neurons, 82%, projected to the ipsilateral PbN, but 85% of the 20 NST neurons tested received synaptic input from the ipsilateral PbN, as did 59% of 22 NST neurons that did not project to the PbN; and 2) although few, 3%, of 119 NST cells projected to the contralateral PbN and 38% received synaptic input from the contralateral PbN. These results demonstrated that taste neurons in the NST not only project to, but also receive descending input from the bilateral PbN and that gustatory neurons in the NST and PbN also communicate with the corresponding nucleus on the contralateral side.
Figures


References
-
- Baird JP, Travers JB, Travers SP. Parametric analysis of gastric distension responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1568–R1580, 2001a - PubMed
-
- Baird JP, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1581–R1593, 2001b - PubMed
-
- Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res 557: 265–279, 1991 - PubMed
-
- Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 383: 245–281, 1997 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

