Placental-fetal glucose exchange and fetal glucose metabolism
- PMID: 18528484
- PMCID: PMC1500912
Placental-fetal glucose exchange and fetal glucose metabolism
Abstract
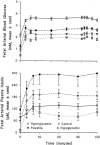
Fetal glucose metabolism depends on additive effects of fetal plasma glucose and insulin. Glucose-stimulated insulin secretion increases over gestation, is down-regulated by constant hyperglycemia, but enhanced by pulsatile hyperglycemia. Insulin production is diminished in fetuses with intrauterine growth restriction (IUGR) by inhibition of pancreatic beta-cell replication, but not by mechanisms that regulate insulin production or secretion, while the opposite occurs with hypoglycemia alone, despite its common occurrence in IUGR. Chronic hyperglycemia down-regulates glucose tolerance and insulin sensitivity with decreased expression of skeletal muscle and hepatic Glut 1 and 4 glucose transporters, while chronic hypoglycemia up-regulates these transporters. The opposite occurs for signal transduction proteins that regulate amino acid synthesis into protein. These results demonstrate the mixed phenotype of the IUGR fetus with enhanced glucose utilization capacity, but diminished protein synthesis and growth. Such adaptations might underlie childhood and adult metabolic disorders of insulin resistance, obesity, and diabetes mellitus.
Figures
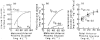

References
-
- Battaglia FC, Meschia G. Orlando: Academic Press; 1986. An Introduction to Fetal Physiology.
-
- Hay WW., Jr Energy and substrate requirements of the placenta and fetus.. Proc Nutr Soc; 1991. pp. 321–336. - PubMed
-
- Hay WW, Jr, Sparks JW, Quissell BJ, Battaglia FC, Meschia G. Simultaneous measurements of umbilical uptake, fetal utilization rate, and fetal turnover rate of glucose. Am J Physiol. 1981;240(6):E662–E668. - PubMed
-
- Hay WW, Jr, Sparks JW, Wilkening RB, Battaglia FC, Meschia G. Fetal glucose uptake and utilization as functions of maternal glucose concentration. Am J Physiol. 1984;246(3 Pt 1):E237–E242. - PubMed
-
- Johnson LW, Smith CH. Monosaccharide transport across microvillous membrane of human placenta. Am J Physiol. 1980;238(5):C160–C168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
