Synthetic growth phenotypes of Escherichia coli lacking ppGpp and transketolase A (tktA) are due to ppGpp-mediated transcriptional regulation of tktB
- PMID: 18532980
- PMCID: PMC2561915
- DOI: 10.1111/j.1365-2958.2008.06317.x
Synthetic growth phenotypes of Escherichia coli lacking ppGpp and transketolase A (tktA) are due to ppGpp-mediated transcriptional regulation of tktB
Abstract
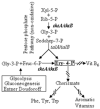
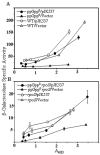
Many physiological adjustments to nutrient changes involve ppGpp. Recent attempts to deduce ppGpp regulatory effects using proteomics or gene profiling can rigorously identify proteins or transcripts, but the functional significance is often unclear. Using a random screen for synthetic lethals we found a ppGpp-dependent functional pathway that operates through transketolase B (TktB), and which is 'buffered' in wildtype strain by the presence of an isozyme, transketolase A (TktA). Transketolase activity is required in cells to make erythrose-4-phosphate, a precursor of aromatic amino acids and vitamins. By studying tktB-dependent nutritional requirements as well as measuring activities using PtalA-tktB'-lacZ transcriptional reporter fusion, we show positive transcriptional regulation of the talA-tktB operon by ppGpp. Our results show the existence of RpoS-dependent and RpoS-independent modes of positive regulation by ppGpp. Both routes of activation are magnified by elevating ppGpp levels with a spoT mutation (spoT-R39A) defective in hydrolase but not synthetase activity or with the stringent suppressor mutations rpoB-A532Delta or rpoB-T563P in the absence of ppGpp.
Figures





References
-
- Barker MM, Gaal T, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. II. Models for positive control based on properties of RNAP mutants and competition for RNAP. J Mol Biol. 2001;305:689–702. - PubMed
-
- Battesti A, Bouveret E. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol Microbiol. 2006;62:1048–1063. - PubMed
-
- Braeken K, Moris M, Daniels R, Vanderleyden J, Michiels J. New horizons for (p)ppGpp in bacterial and plant physiology. Trends Microbiol. 2006;14:45–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

