The prodomain of Toxoplasma gondii GPI-anchored subtilase TgSUB1 mediates its targeting to micronemes
- PMID: 18532988
- PMCID: PMC3556455
- DOI: 10.1111/j.1600-0854.2008.00774.x
The prodomain of Toxoplasma gondii GPI-anchored subtilase TgSUB1 mediates its targeting to micronemes
Abstract
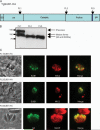
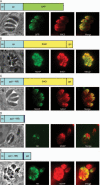
Subtilisin-like proteases have been proposed to play an important role for parasite survival in Toxoplasma gondii (Tg) and Plasmodium falciparum. The T. gondii subtilase TgSUB1 is located in the microneme, an apical secretory organelle whose contents mediate adhesion to the host during invasion. TgSUB1 is predicted to contain a glycosyl-phosphatidylinositol (GPI) anchor. This is unusual as Toxoplasma GPI-anchored proteins are targeted to the parasite's surface. In this study, we report that the subtilase TgSUB1 is indeed a GPI-anchored protein but contains dominant microneme targeting signals. Accurate targeting of TgSUB1 to the micronemes is dependent upon several factors including promoter strength and timing, accurate processing and folding. We analyzed the targeting domains of TgSUB1 using TgSUB1 deletion constructs and chimeras made between TgSUB1 and reporter proteins. The TgSUB1 prodomain is responsible for trafficking to the micronemes and is sufficient for targeting a reporter protein to the micronemes. Trafficking is dependent upon correct folding or other context-dependent conformation as the prodomain expressed alone is unable to reach the micromenes. Therefore, TgSUB1 is a novel example of a GPI-anchored protein in T. gondii that bypasses the GPI-dependent surface trafficking pathway to traffic to micronemes, specialized regulated secretory organelles.
Figures





Similar articles
-
Toxoplasma gondii protease TgSUB1 is required for cell surface processing of micronemal adhesive complexes and efficient adhesion of tachyzoites.Cell Microbiol. 2010 Dec;12(12):1792-808. doi: 10.1111/j.1462-5822.2010.01509.x. Cell Microbiol. 2010. PMID: 20678172 Free PMC article.
-
A conserved subtilisin-like protein TgSUB1 in microneme organelles of Toxoplasma gondii.J Biol Chem. 2001 Nov 30;276(48):45341-8. doi: 10.1074/jbc.M106665200. Epub 2001 Sep 19. J Biol Chem. 2001. PMID: 11564738
-
Preparing for an invasion: charting the pathway of adhesion proteins to Toxoplasma micronemes.Parasitol Res. 2006 Apr;98(5):389-95. doi: 10.1007/s00436-005-0062-2. Epub 2005 Dec 30. Parasitol Res. 2006. PMID: 16385407 Review.
-
A transient forward-targeting element for microneme-regulated secretion in Toxoplasma gondii.Biol Cell. 2008 Apr;100(4):253-64. doi: 10.1042/BC20070076. Biol Cell. 2008. PMID: 17995454 Free PMC article.
-
Biogenesis and secretion of micronemes in Toxoplasma gondii.Cell Microbiol. 2019 May;21(5):e13018. doi: 10.1111/cmi.13018. Epub 2019 Mar 18. Cell Microbiol. 2019. PMID: 30791192 Review.
Cited by
-
Global Analysis of Palmitoylated Proteins in Toxoplasma gondii.Cell Host Microbe. 2015 Oct 14;18(4):501-11. doi: 10.1016/j.chom.2015.09.006. Cell Host Microbe. 2015. PMID: 26468752 Free PMC article.
-
A cathepsin C-like protease post-translationally modifies Toxoplasma gondii secretory proteins for optimal invasion and egress.bioRxiv [Preprint]. 2023 Jan 22:2023.01.21.525043. doi: 10.1101/2023.01.21.525043. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0017423. doi: 10.1128/mbio.00174-23. PMID: 36712013 Free PMC article. Updated. Preprint.
-
Toxoplasma gondii protease TgSUB1 is required for cell surface processing of micronemal adhesive complexes and efficient adhesion of tachyzoites.Cell Microbiol. 2010 Dec;12(12):1792-808. doi: 10.1111/j.1462-5822.2010.01509.x. Cell Microbiol. 2010. PMID: 20678172 Free PMC article.
-
Membrane skeletal association and post-translational allosteric regulation of Toxoplasma gondii GAPDH1.Mol Microbiol. 2017 Feb;103(4):618-634. doi: 10.1111/mmi.13577. Epub 2016 Dec 23. Mol Microbiol. 2017. PMID: 27859784 Free PMC article.
-
A cathepsin C-like protease mediates the post-translation modification of Toxoplasma gondii secretory proteins for optimal invasion and egress.mBio. 2023 Aug 31;14(4):e0017423. doi: 10.1128/mbio.00174-23. Epub 2023 Jun 16. mBio. 2023. PMID: 37326431 Free PMC article.
References
-
- Carruthers V, Sibley L. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. - PubMed
-
- Carruthers V, Boothroyd J. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2006;9:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

