Rapid preparation of nuclei-depleted detergent-resistant membrane fractions suitable for proteomics analysis
- PMID: 18534013
- PMCID: PMC2440737
- DOI: 10.1186/1471-2121-9-30
Rapid preparation of nuclei-depleted detergent-resistant membrane fractions suitable for proteomics analysis
Abstract
Background: Cholesterol-rich membrane microdomains known as lipid rafts have been implicated in diverse physiologic processes including lipid transport and signal transduction. Lipid rafts were originally defined as detergent-resistant membranes (DRMs) due to their relative insolubility in cold non-ionic detergents. Recent findings suggest that, although DRMs are not equivalent to lipid rafts, the presence of a given protein within DRMs strongly suggests its potential for raft association in vivo. Therefore, isolation of DRMs represents a useful starting point for biochemical analysis of lipid rafts. The physicochemical properties of DRMs present unique challenges to analysis of their protein composition. Existing methods of isolating DRM-enriched fractions involve flotation of cell extracts in a sucrose density gradient, which, although successful, can be labor intensive, time consuming and results in dilute sucrose-containing fractions with limited utility for direct proteomic analysis. In addition, several studies describing the proteomic characterization of DRMs using this and other approaches have reported the presence of nuclear proteins in such fractions. It is unclear whether these results reflect trafficking of nuclear proteins to DRMs or whether they arise from nuclear contamination during isolation. To address these issues, we have modified a published differential detergent extraction method to enable rapid DRM isolation that minimizes nuclear contamination and yields fractions compatible with mass spectrometry.
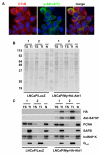
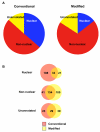
Results: DRM-enriched fractions isolated using the conventional or modified extraction methods displayed comparable profiles of known DRM-associated proteins, including flotillins, GPI-anchored proteins and heterotrimeric G-protein subunits. Thus, the modified procedure yielded fractions consistent with those isolated by existing methods. However, we observed a marked reduction in the percentage of nuclear proteins identified in DRM fractions isolated with the modified method (15%) compared to DRMs isolated by conventional means (36%). Furthermore, of the 21 nuclear proteins identified exclusively in modified DRM fractions, 16 have been reported to exist in other subcellular sites, with evidence to suggest shuttling of these species between the nucleus and other organelles.
Conclusion: We describe a modified DRM isolation procedure that generates DRMs that are largely free of nuclear contamination and that is compatible with downstream proteomic analyses with minimal additional processing. Our findings also imply that identification of nuclear proteins in DRMs is likely to reflect legitimate movement of proteins between compartments, and is not a result of contamination during extraction.
Figures
Similar articles
-
Analysis of raft affinity of membrane proteins by detergent-insolubility.Methods Mol Biol. 2007;398:9-20. doi: 10.1007/978-1-59745-513-8_2. Methods Mol Biol. 2007. PMID: 18214370
-
Using NK Cell Lipid Raft Fractionation to Understand the Role of Lipid Rafts in NK Cell Receptor Signaling.Methods Mol Biol. 2016;1441:131-9. doi: 10.1007/978-1-4939-3684-7_11. Methods Mol Biol. 2016. PMID: 27177662
-
Detergent-free domain isolated from Xenopus egg plasma membrane with properties similar to those of detergent-resistant membranes.Biochemistry. 2002 Nov 5;41(44):13189-97. doi: 10.1021/bi026107b. Biochemistry. 2002. PMID: 12403620
-
Membrane rafts of the human red blood cell.Mol Membr Biol. 2014 Mar-May;31(2-3):47-57. doi: 10.3109/09687688.2014.896485. Epub 2014 Apr 10. Mol Membr Biol. 2014. PMID: 24720522 Review.
-
Lipid rafts, detergent-resistant membranes, and raft targeting signals.Physiology (Bethesda). 2006 Dec;21:430-9. doi: 10.1152/physiol.00032.2006. Physiology (Bethesda). 2006. PMID: 17119156 Review.
Cited by
-
Proteomic Analysis of Epithelial to Mesenchymal Transition (EMT) Reveals Cross-talk between SNAIL and HDAC1 Proteins in Breast Cancer Cells.Mol Cell Proteomics. 2016 Mar;15(3):906-17. doi: 10.1074/mcp.M115.052910. Epub 2016 Jan 13. Mol Cell Proteomics. 2016. PMID: 26764010 Free PMC article.
-
Heterotrimeric G-proteins interact directly with cytoskeletal components to modify microtubule-dependent cellular processes.Neurosignals. 2009;17(1):100-8. doi: 10.1159/000186693. Epub 2009 Feb 12. Neurosignals. 2009. PMID: 19212143 Free PMC article. Review.
-
Paracrine Fibroblast Growth Factor Initiates Oncogenic Synergy with Epithelial FGFR/Src Transformation in Prostate Tumor Progression.Neoplasia. 2018 Mar;20(3):233-243. doi: 10.1016/j.neo.2018.01.006. Epub 2018 Feb 11. Neoplasia. 2018. PMID: 29444487 Free PMC article.
-
Identification of novel γ-secretase-associated proteins in detergent-resistant membranes from brain.J Biol Chem. 2012 Apr 6;287(15):11991-2005. doi: 10.1074/jbc.M111.246074. Epub 2012 Feb 7. J Biol Chem. 2012. PMID: 22315232 Free PMC article.
-
Regulation of the V-ATPase along the endocytic pathway occurs through reversible subunit association and membrane localization.PLoS One. 2008 Jul 23;3(7):e2758. doi: 10.1371/journal.pone.0002758. PLoS One. 2008. PMID: 18648502 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

