Identification of glycosaminoglycan binding regions in the Plasmodium falciparum encoded placental sequestration ligand, VAR2CSA
- PMID: 18534039
- PMCID: PMC2430714
- DOI: 10.1186/1475-2875-7-104
Identification of glycosaminoglycan binding regions in the Plasmodium falciparum encoded placental sequestration ligand, VAR2CSA
Abstract
Background: Pregnancy malaria is caused by Plasmodium falciparum-infected erythrocytes binding the placental receptor chondroitin sulfate A (CSA). This results in accumulation of parasites in the placenta with severe clinical consequences for the mother and her unborn child. Women become resistant to placental malaria as antibodies are acquired which specifically target the surface of infected erythrocytes binding in the placenta. VAR2CSA is most likely the parasite-encoded protein which mediates binding to the placental receptor CSA. Several domains have been shown to bind CSA in vitro; and it is apparent that a VAR2CSA-based vaccine cannot accommodate all the CSA binding domains and serovariants. It is thus of high priority to define minimal ligand binding regions throughout the VAR2CSA molecule.
Methods: To define minimal CSA-binding regions/peptides of VAR2CSA, a phage display library based on the entire var2csa coding region was constructed. This library was screened on immobilized CSA and cells expressing CSA resulting in a limited number of CSA-binding phages. Antibodies against these peptides were affinity purified and tested for reactivity against CSA-binding infected erythrocytes.
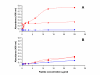
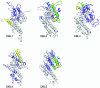
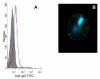
Results: The most frequently identified phages expressed peptides residing in the parts of VAR2CSA previously defined as CSA binding. In addition, most of the binding regions mapped to surface-exposed parts of VAR2CSA. The binding of a DBL2X peptide to CSA was confirmed with a synthetic peptide. Antibodies against a CSA-binding DBL2X peptide reacted with the surface of infected erythrocytes indicating that this epitope is accessible for antibodies on native VAR2CSA on infected erythrocytes.
Conclusion: Short continuous regions of VAR2CSA with affinity for multiple types of CSA were defined. A number of these regions localize to CSA-binding domains and to surface-exposed regions within these domains and a synthetic peptide corresponding to a peptide sequence in DBL2 was shown to bind to CSA and not to CSC. It is likely that some of these epitopes are involved in native parasite CSA adhesion. However, antibodies directed against single epitopes did not inhibit parasite adhesion. This study supports phage display as a technique to identify CSA-binding regions of large proteins such as VAR2CSA.
Figures

Similar articles
-
Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria.J Biol Chem. 2012 Jul 6;287(28):23332-45. doi: 10.1074/jbc.M112.348839. Epub 2012 May 8. J Biol Chem. 2012. PMID: 22570492 Free PMC article.
-
A single full-length VAR2CSA ectodomain variant purifies broadly neutralizing antibodies against placental malaria isolates.Elife. 2022 Feb 1;11:e76264. doi: 10.7554/eLife.76264. Elife. 2022. PMID: 35103596 Free PMC article.
-
Characterization of anti-var2CSA-PfEMP1 cytoadhesion inhibitory mouse monoclonal antibodies.Microbes Infect. 2006 Nov-Dec;8(14-15):2863-71. doi: 10.1016/j.micinf.2006.09.005. Epub 2006 Oct 17. Microbes Infect. 2006. PMID: 17095277
-
Designing a VAR2CSA-based vaccine to prevent placental malaria.Vaccine. 2015 Dec 22;33(52):7483-8. doi: 10.1016/j.vaccine.2015.10.011. Epub 2015 Nov 26. Vaccine. 2015. PMID: 26469717 Free PMC article. Review.
-
Can any lessons be learned from the ambiguous glycan binding of PfEMP1 domains?Trends Parasitol. 2010 May;26(5):230-5. doi: 10.1016/j.pt.2010.02.002. Epub 2010 Feb 26. Trends Parasitol. 2010. PMID: 20189879 Review.
Cited by
-
Molecular Principles of Intrauterine Growth Restriction in Plasmodium Falciparum Infection.Front Endocrinol (Lausanne). 2019 Mar 1;10:98. doi: 10.3389/fendo.2019.00098. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30930847 Free PMC article. Review.
-
Targeted delivery of doxorubicin by CSA-binding nanoparticles for choriocarcinoma treatment.Drug Deliv. 2018 Nov;25(1):461-471. doi: 10.1080/10717544.2018.1435750. Drug Deliv. 2018. PMID: 29426237 Free PMC article.
-
Oncofetal Chondroitin Sulfate: A Putative Therapeutic Target in Adult and Pediatric Solid Tumors.Cells. 2020 Mar 28;9(4):818. doi: 10.3390/cells9040818. Cells. 2020. PMID: 32231047 Free PMC article. Review.
-
Insight into antigenic diversity of VAR2CSA-DBL5ε domain from multiple Plasmodium falciparum placental isolates.PLoS One. 2010 Oct 1;5(10):e13105. doi: 10.1371/journal.pone.0013105. PLoS One. 2010. PMID: 20957045 Free PMC article.
-
Antibodies to Cryptic Epitopes in Distant Homologues Underpin a Mechanism of Heterologous Immunity between Plasmodium vivax PvDBP and Plasmodium falciparum VAR2CSA.mBio. 2019 Oct 8;10(5):e02343-19. doi: 10.1128/mBio.02343-19. mBio. 2019. PMID: 31594821 Free PMC article.
References
-
- Ricke CH, Staalsoe T, Koram K, Akanmori BD, Riley EM, Theander TG, Hviid L. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol. 2000;165:3309–3316. - PubMed
-
- Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

