N-terminal processing of proteins exported by malaria parasites
- PMID: 18534695
- PMCID: PMC2922945
- DOI: 10.1016/j.molbiopara.2008.04.011
N-terminal processing of proteins exported by malaria parasites
Abstract
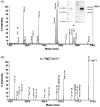
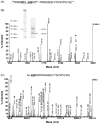
Malaria parasites utilize a short N-terminal amino acid motif termed the Plasmodium export element (PEXEL) to export an array of proteins to the host erythrocyte during blood stage infection. Using immunoaffinity chromatography and mass spectrometry, insight into this signal-mediated trafficking mechanism was gained by discovering that the PEXEL motif is cleaved and N-acetylated. PfHRPII and PfEMP2 are two soluble proteins exported by Plasmodium falciparum that were demonstrated to undergo PEXEL cleavage and N-acetylation, thus indicating that this N-terminal processing may be general to many exported soluble proteins. It was established that PEXEL processing occurs upstream of the brefeldin A-sensitive trafficking step in the P. falciparum secretory pathway, therefore cleavage and N-acetylation of the PEXEL motif occurs in the endoplasmic reticulum (ER) of the parasite. Furthermore, it was shown that the recognition of the processed N-terminus of exported proteins within the parasitophorous vacuole may be crucial for protein transport to the host erythrocyte. It appears that the PEXEL may be defined as a novel ER peptidase cleavage site and a classical N-acetyltransferase substrate sequence.
Figures

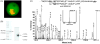
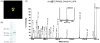
References
-
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–9. - PubMed
-
- Kirk K. Membrane transport in the malaria-infected erythrocyte. Physiol Rev. 2001;81:495–537. - PubMed
-
- Pologe LG, Ravetch JV. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. Nature. 1986;322:474–7. - PubMed
-
- Baruch DI, Pasloske BL, Singh HB, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

