New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice
- PMID: 18534740
- PMCID: PMC2562461
- DOI: 10.1016/j.mce.2008.04.003
New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice
Abstract
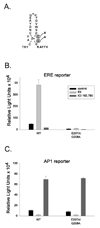
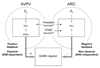
Estrogen receptor alpha (ERalpha) mediates estrogen (E2) actions in the brain and is critical for normal reproductive function and behavior. In the classical pathway, ERalpha binds to estrogen response elements (EREs) to regulate gene transcription. ERalpha can also participate in several non-classical pathways, including ERE-independent gene transcription via protein-protein interactions with transcription factors and rapid, non-genotropic pathways. To distinguish between ERE-dependent and ERE-independent mechanisms of E2 action in vivo, we have created ERalpha null mice that possess an ER knock-in mutation (E207A/G208A; "AA"), in which the mutant ERalpha cannot bind to DNA but retains activity in ERE-independent pathways (ERalpha(-/AA) mice). Understanding the molecular mechanisms of ERalpha action will be helpful in developing pharmacological therapies that differentiate between ERE-dependent and ERE-independent processes. This review focuses on how the ERalpha(-/AA) model has contributed to our knowledge of ERalpha signaling mechanisms in estrogen regulation of the reproductive axis and sexual behavior.
Figures



References
-
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144:84–93. - PubMed
-
- Arreguin-Arevalo JA, Nett TM. A nongenomic action of estradiol as the mechanism underlying the acute suppression of secretion of luteinizing hormone in ovariectomized ewes. Biol Reprod. 2006;74:202–208. - PubMed
-
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. - PubMed
-
- Bodo C, Kudwa AE, Rissman EF. Both estrogen receptor-alpha and -beta are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology. 2006;147:415–420. - PubMed
-
- Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

