Histone chaperones in nucleosome eviction and histone exchange
- PMID: 18534842
- PMCID: PMC2525571
- DOI: 10.1016/j.sbi.2008.04.003
Histone chaperones in nucleosome eviction and histone exchange
Abstract
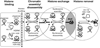
The recent two years have led to the realization that histone chaperones contribute to the delicate balance between nucleosome assembly and re-assembly during transcription, and may in fact be involved as much in histone eviction as they are in chromatin assembly. Recent structural studies (in particular, the structure of an Asf1-H3/H4 complex) have suggested mechanisms by which this may be accomplished. The incorporation of various histone variants into nucleosomes has diverse effects on nucleosome structure, stability, and the ability of nucleosomal arrays to condense into chromatin higher order structures. It is likely that these seemingly independent ways to modify chromatin structure are interdependent.
Figures



References
-
- Luger K, Maeder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251–259. - PubMed
-
- De Koning L, Corpet A, Haber JE, Almouzni G. Histone chaperones: an escort network regulating histone traffic. Nat Struct Mol Biol. 2007;14:997–1007. - PubMed
-
- Boulard M, Bouvet P, Kundu TK, Dimitrov S. Histone variant nucleosomes: structure, function and implication in disease. Subcell Biochem. 2007;41:71–89. - PubMed
-
- Luger K. Dynamic nucleosomes. Chromosome Res. 2006;14:5–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

