Structure of the human 26S proteasome: subunit radial displacements open the gate into the proteolytic core
- PMID: 18534977
- PMCID: PMC2517000
- DOI: 10.1074/jbc.M802716200
Structure of the human 26S proteasome: subunit radial displacements open the gate into the proteolytic core
Abstract
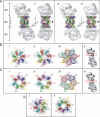
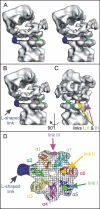
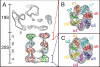
The 26S proteasome plays an essential role in regulating many cellular processes by the degradation of proteins targeted for breakdown by ubiquitin conjugation. The 26S complex is formed from the 20S core, which contains the proteolytic active sites, and 19S regulatory complexes, which bind to the 20S core to activate it and confer specificity for ubiquitinated protein substrates. We have determined the structure of the human 26S proteasome by electron microscopy and single particle analysis. In our reconstructions the crystallographic structure of each of the subunits of the 20S core can be unambiguously docked by direct recognition of each of their densities. Our results show for the first time that binding of the 19S regulatory particle results in the radial displacement of the adjacent subunits of the 20S core leading to opening of a wide channel into the proteolytic chamber. The analysis of a proteasome complex formed from one 20S core with a single 19S regulatory particle attached serve as control to our observations. We suggest locations for some of the 19S regulatory particle subunits.
Figures

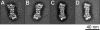


References
-
- Goldberg, A. L. (2003) Nature 426 895-899 - PubMed
-
- Löwe, J., Stock, D., Jap, B., Zwickl, P., Baumeister, W., and Huber, R. (1995) Science 268 533-539 - PubMed
-
- Groll, M., Ditzel, L., Lowe, J., Stock, D., Bochtler, M., Bartunik, H. D., and Huber, R. (1997) Nature 386 463-471 - PubMed
-
- Groll, M., Bajorek, M., Köhler, A., Moroder, L., Rubin, D. M., Huber, R., Glickman, M. H., and Finley, D. (2000) Nat. Struct. Biol. 7 1062-1067 - PubMed
-
- Kohler, A., Bajorek, M., Groll, M., Moroder, L., Rubin, D. M., Huber, R., Glickman, M. H., and Finley, D. (2001) Biochimie (Paris) 83 325-332 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

