Rhesus monkeys and humans share common susceptibility genes for age-related macular disease
- PMID: 18535016
- PMCID: PMC2733804
- DOI: 10.1093/hmg/ddn167
Rhesus monkeys and humans share common susceptibility genes for age-related macular disease
Abstract
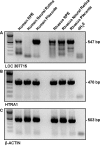
Age-related macular degeneration (AMD), a complex multigenic disorder and the most common cause of vision loss in the elderly, is associated with polymorphisms in the LOC387715/ARMS2 and HTRA1 genes on 10q26. Like humans, macaque monkeys possess a macula and develop age-related macular pathologies including drusen, the phenotypic hallmark of AMD. We genotyped a cohort of 137 unrelated rhesus macaques with and without macular drusen. As in humans, one variant within LOC387715/ARMS2 and one in HTRA1 were significantly associated with affected status. HTRA1 and the predicted LOC387715/ARMS2 gene were both transcribed in rhesus and human retina and retinal pigment epithelium. Among several primate species, orthologous exons for the human LOC387715/ARMS2 gene were present only in Old World monkeys and apes. In functional analyses, the disease-associated HTRA1 polymorphism resulted in a 2-fold increase in gene expression, supporting a role in pathogenesis. These results demonstrate that two genes associated with AMD in humans are also associated with macular disease in rhesus macaques and that one of these genes is specific to higher primates. This is the first evidence that humans and macaques share the same genetic susceptibility factors for a common complex disease.
Figures



References
-
- Klein R., Klein B.E., Knudtson M.D., Meuer S.M., Swift M., Gangnon R.E. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. - PubMed
-
- Klein M.L., Francis P.J. Genetics of age-related macular degeneration. Ophthalmol. Clin. North Am. 2003;16:567–574. - PubMed
-
- Maller J., George S., Purcell S., Fagerness J., Altshuler D., Daly M.J., Seddon J.M. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat. Genet. 2006;38:1055–1059. - PubMed
-
- Francis P.J., George S., Schultz D.W., Rosner B., Hamon S., Ott J., Weleber R.G., Klein M.L., Seddon J.M. The LOC387715 gene, smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum. Hered. 2007;63:212–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

