Tegument proteins of human cytomegalovirus
- PMID: 18535146
- PMCID: PMC2415745
- DOI: 10.1128/MMBR.00040-07
Tegument proteins of human cytomegalovirus
Abstract
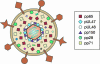
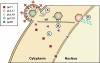
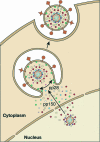
Human cytomegalovirus (HCMV) is a common, medically relevant human herpesvirus. The tegument layer of herpesvirus virions lies between the genome-containing capsids and the viral envelope. Proteins within the tegument layer of herpesviruses are released into the cell upon entry when the viral envelope fuses with the cell membrane. These proteins are fully formed and active and control viral entry, gene expression, and immune evasion. Most tegument proteins accumulate to high levels during later stages of infection, when they direct the assembly and egress of progeny virions. Thus, viral tegument proteins play critical roles at the very earliest and very last steps of the HCMV lytic replication cycle. This review summarizes HCMV tegument composition and structure as well as the known and speculated functions of viral tegument proteins. Important directions for future investigation and the challenges that lie ahead are identified and discussed.
Figures

References
-
- Adair, R., E. R. Douglas, J. B. Maclean, S. Y. Graham, J. D. Aitken, F. E. Jamieson, and D. J. Dargan. 2002. The products of human cytomegalovirus genes UL23, UL24, UL43 and US22 are tegument components. J. Gen. Virol. 831315-1324. - PubMed
-
- Agromayor, M., and J. Martin-Serrano. 2006. Interaction of AMSH with ESCRT-III and deubiquitination of endosomal cargo. J. Biol. Chem. 28123083-23091. - PubMed
-
- Andoniou, C. E., and M. A. Degli-Esposti. 2006. Insights into the mechanisms of CMV-mediated interference with cellular apoptosis. Immunol. Cell Biol. 8499-106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

