Structure, function, and evolution of bacterial ATP-binding cassette systems
- PMID: 18535149
- PMCID: PMC2415747
- DOI: 10.1128/MMBR.00031-07
Structure, function, and evolution of bacterial ATP-binding cassette systems
Abstract
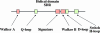
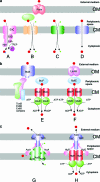
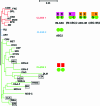
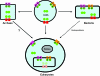
ATP-binding cassette (ABC) systems are universally distributed among living organisms and function in many different aspects of bacterial physiology. ABC transporters are best known for their role in the import of essential nutrients and the export of toxic molecules, but they can also mediate the transport of many other physiological substrates. In a classical transport reaction, two highly conserved ATP-binding domains or subunits couple the binding/hydrolysis of ATP to the translocation of particular substrates across the membrane, through interactions with membrane-spanning domains of the transporter. Variations on this basic theme involve soluble ABC ATP-binding proteins that couple ATP hydrolysis to nontransport processes, such as DNA repair and gene expression regulation. Insights into the structure, function, and mechanism of action of bacterial ABC proteins are reported, based on phylogenetic comparisons as well as classic biochemical and genetic approaches. The availability of an increasing number of high-resolution structures has provided a valuable framework for interpretation of recent studies, and realistic models have been proposed to explain how these fascinating molecular machines use complex dynamic processes to fulfill their numerous biological functions. These advances are also important for elucidating the mechanism of action of eukaryotic ABC proteins, because functional defects in many of them are responsible for severe human inherited diseases.
Figures








References
-
- Akama, H., M. Kanemaki, M. Yoshimura, T. Tsukihara, T. Kashiwagi, H. Yoneyama, S. Narita, A. Nakagawa, and T. Nakae. 2004. Crystal structure of the drug discharge outer membrane protein, OprM, of Pseudomonas aeruginosa: dual modes of membrane anchoring and occluded cavity end. J. Biol. Chem. 27952816-52819. - PubMed
-
- Akama, H., T. Matsuura, S. Kashiwagi, H. Yoneyama, S. Narita, T. Tsukihara, A. Nakagawa, and T. Nakae. 2004. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J. Biol. Chem. 27925939-25942. - PubMed
-
- Albers, S. V., S. M. Koning, W. N. Konings, and A. J. Driessen. 2004. Insights into ABC transport in archaea. J. Bioenerg. Biomembr. 365-15. - PubMed
-
- Alekshun, M. N., and S. B. Levy. 2007. Molecular mechanisms of antibacterial multidrug resistance. Cell 1281037-1050. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

