Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans
- PMID: 18535579
- PMCID: PMC3697853
- DOI: 10.1038/nm.f.1759
Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans
Abstract
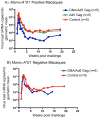
The adenovirus type 5 (Ad5)-based vaccine developed by Merck failed to either prevent HIV-1 infection or suppress viral load in subsequently infected subjects in the STEP human Phase 2b efficacy trial. Analogous vaccines had previously also failed in the simian immunodeficiency virus (SIV) challenge-rhesus macaque model. In contrast, vaccine protection studies that used challenge with a chimeric simian-human immunodeficiency virus (SHIV89.6P) in macaques did not predict the human trial results. Ad5 vector-based vaccines did not protect macaques from infection after SHIV89.6P challenge but did cause a substantial reduction in viral load and a preservation of CD4+ T cell counts after infection, findings that were not reproduced in the human trials. Although the SIV challenge model is incompletely validated, we propose that its expanded use can help facilitate the prioritization of candidate HIV-1 vaccines, ensuring that resources are focused on the most promising candidates. Vaccine designers must now develop T cell vaccine strategies that reduce viral load after heterologous challenge.
Figures


References
-
- Baba TW, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. - PubMed
-
- Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. - PubMed
-
- Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. - PubMed
-
- VaxGen. Press Release. 2003. VaxGen Announces Results of its Phase III HIV Vaccine Trial in Thailand: Vaccine Fails to Meet Endpoints.
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI45463/AI/NIAID NIH HHS/United States
- R01 AI052056/AI/NIAID NIH HHS/United States
- R37 AI036082/AI/NIAID NIH HHS/United States
- R37 AI36082/AI/NIAID NIH HHS/United States
- R24 RR015371/RR/NCRR NIH HHS/United States
- R37 AI033292/AI/NIAID NIH HHS/United States
- R01 AI049120/AI/NIAID NIH HHS/United States
- R01 AI045463/AI/NIAID NIH HHS/United States
- R37 AI33292/AI/NIAID NIH HHS/United States
- U01 AI69420/AI/NIAID NIH HHS/United States
- R01 AI055332/AI/NIAID NIH HHS/United States
- U01 AI069420/AI/NIAID NIH HHS/United States
- R24 RR016038/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

