Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice
- PMID: 18535669
- PMCID: PMC2413184
- DOI: 10.1172/JCI33493
Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice
Abstract
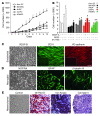
Infantile hemangioma is a benign endothelial tumor composed of disorganized blood vessels. It exhibits a unique life cycle of rapid postnatal growth followed by slow regression to a fibrofatty residuum. Here, we have reported the isolation of multipotential stem cells from hemangioma tissue that give rise to hemangioma-like lesions in immunodeficient mice. Cells were isolated based on expression of the stem cell marker CD133 and expanded from single cells as clonal populations. The CD133-selected cells generated human blood vessels 7 days after implantation in immunodeficient mice. Cell retrieval experiments showed the cells could again form vessels when transplanted into secondary recipients. The human vessels expressed GLUT-1 and merosin, immunodiagnostic markers for infantile hemangioma. Two months after implantation, the number of blood vessels diminished and human adipocytes became evident. Lentiviral expression of GFP was used to confirm that the hemangioma-derived cells formed the blood vessels and adipocytes in the immunodeficient mice. Thus, when transplanted into immunodeficient mice, hemangioma-derived cells recapitulated the unique evolution of infantile hemangioma--the formation of blood vessels followed by involution to fatty tissue. In summary, this study identifies a stem cell as the cellular origin of infantile hemangioma and describes for what we believe is the first time an animal model for this common tumor of infancy.
Figures




References
-
- Bielenberg D.R., et al. Progressive growth of infantile cutaneous hemangiomas is directly correlated with hyperplasia and angiogenesis of adjacent epidermis and inversely correlated with expression of the endogenous angiogenesis inhibitor, IFN-beta. Int. J. Oncol. 1999;14:401–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

