On the fitting of binding data when receptor dimerization is suspected
- PMID: 18536745
- PMCID: PMC2527846
- DOI: 10.1038/bjp.2008.234
On the fitting of binding data when receptor dimerization is suspected
Abstract
Mechanistic and empirical modelling are compared in context of dimeric receptors. In particular, the supposed advantages of the two-state dimer model for fitting of binding data with respect to classical approaches such as the two-independent sites model are investigated. The two models are revisited from both the mechanistic and empirical point of views. The problem of overparameterized models and the benefits of the concurrent use of mechanistic and empirical models for mechanism analysis are discussed. The pros and cons of mathematical models are examined with special emphasis given to the interpretation of the connection between the shapes of the curves and receptor cooperativity. It is shown that a given pharmacological phenotype (curve shape) can be obtained from different receptor genotypes (as, for instance, non-interconvertible monomeric receptor species, receptor-G protein interactions and dimeric receptors), though values of the Hill coefficient greater than one are indicative of receptor oligomerization. The existence of a relationship between the recently defined dimer cooperativity index and the more familiar Hill coefficient is proven.
Figures
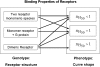
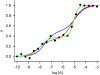
 , both lower and equal to one, can be obtained from either of the genotypes (non-interconvertible monomeric receptor species; receptor-G protein interaction, where the concentration of the G protein is limited; and a dimeric receptor); however, values greater than one are indicative of receptor oligomerization.
, both lower and equal to one, can be obtained from either of the genotypes (non-interconvertible monomeric receptor species; receptor-G protein interaction, where the concentration of the G protein is limited; and a dimeric receptor); however, values greater than one are indicative of receptor oligomerization.
References
-
- Albizu L, Balestre MN, Breton C, Pin JP, Manning M, Mouillac B, et al. Probing the existence of G protein-coupled receptor dimers by positive and negative ligand-dependent cooperative binding. Mol Pharmacol. 2006;70:1783–1791. - PubMed
-
- Armstrong D, Strange PG. Dopamine D2 receptor dimer formation: evidence from ligand binding. J Biol Chem. 2001;276:22621–22629. - PubMed
-
- Casadó V, Cortés A, Ciruela F, Mallol J, Ferré S, Lluis C, et al. Old and new ways to calculate the affinity of agonists and antagonists interacting with G-protein-coupled monomeric and dimeric receptors: the receptor-dimer cooperativity index. Pharmacol Ther. 2007;116:343–354. - PubMed
-
- Chidiac P, Green MA, Pawagi AB, Wells JW. Cardiac muscarinic receptors. Cooperativity as the basis for multiple states of affinity. Biochemistry. 1997;36:7361–7379. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

