Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla
- PMID: 18537121
- PMCID: PMC2481516
- DOI: 10.1002/cne.21757
Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla
Abstract
Understanding the visual pathways of the fly's compound eye has been blocked for decades at the second optic neuropil, the medulla, a two-part relay comprising 10 strata (M1-M10), and the largest neuropil in the fly's brain. Based on the modularity of its composition, and two previous reports, on Golgi-impregnated cell types (Fischbach and Dittrich, Cell Tissue Res.,1989; 258:441-475) and their synaptic circuits in the first neuropil, the lamina, we used serial-section electron microscopy to examine inputs to the distal strata M1-M6. We report the morphology of the reconstructed medulla terminals of five lamina cells, L1-L5, two photoreceptors, R7 and R8, and three neurons, medulla cell T1 and centrifugal cells C2 and C3. The morphology of these conforms closely to previous reports from Golgi impregnation. This fidelity provides assurance that our reconstructions are complete and accurate. Synapses of these terminals broadly localize to the terminal and provide contacts to unidentified targets, mostly medulla cells, as well as sites of connection between the terminals themselves. These reveal that R8 forms contacts upon R7 and thus between these two spectral inputs; that L3 provides input upon both pathways, adding an achromatic input; that the terminal of L5 reciprocally connects to that of L1, thus being synaptic in the medulla despite lacking synapses in the lamina; that the motion-sensing input cells L1 and L2 lack direct interconnection but both receive input from C2 and C3, resembling lamina connections of these cells; and that, as in the lamina, T1 provides no output chemical synapses.
(c) 2008 Wiley-Liss, Inc.
Figures




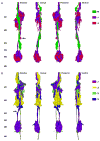

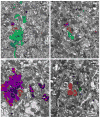
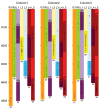
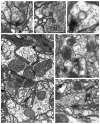


References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Bausenwein B, Fischbach K-F. Activity labeling patterns in the medulla of Drosophila melanogaster caused by motion stimuli. Cell Tiss Res. 1992;270:25–35. - PubMed
-
- Bausenwein B, Dittrich APM, Fischbach K-F. The optic lobe of Drosophila melanogaster II. Sorting of retinotopic pathways in the medulla. Cell Tissue Res. 1992;267:17–28. - PubMed
-
- Bodian D. The generalized vertebrate neuron. Science. 1962;137:323–326. - PubMed
-
- Borst A. Models of motion detection. Nat Neurosci. 2000;3(Suppl):1168. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

