Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation
- PMID: 18537191
- PMCID: PMC3800167
- DOI: 10.1002/hep.22342
Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation
Abstract
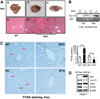
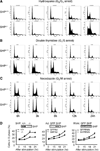
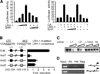
The small heterodimer partner (SHP; NROB2), a member of the nuclear receptor superfamily, contributes to the biological regulation of several major functions of the liver. However, the role of SHP in cellular proliferation and tumorigenesis has not been investigated before. Here we report that SHP negatively regulates tumorigenesis both in vivo and in vitro. SHP-/- mice aged 12 to 15 months old developed spontaneous hepatocellular carcinoma, which was found to be strongly associated with enhanced hepatocyte proliferation and increased cyclin D1 expression. In contrast, overexpressing SHP in hepatocytes of SHP-transgenic mice reversed this effect. Embryonic fibroblasts lacking SHP showed enhanced proliferation and produced increased cyclin D1 messenger RNA and protein, and SHP was shown to be a direct negative regulator of cyclin D1 gene transcription. The immortal SHP-/- fibroblasts displayed characteristics of malignant transformed cells and formed tumors in nude mice.
Conclusion: These results provide first evidence that SHP plays tumor suppressor function by negatively regulating cellular growth.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures


Similar articles
-
Loss of the orphan nuclear receptor SHP is more pronounced in fibrolamellar carcinoma than in typical hepatocellular carcinoma.PLoS One. 2012;7(1):e30944. doi: 10.1371/journal.pone.0030944. Epub 2012 Jan 23. PLoS One. 2012. PMID: 22292081 Free PMC article.
-
Small heterodimer partner overexpression partially protects against liver tumor development in farnesoid X receptor knockout mice.Toxicol Appl Pharmacol. 2013 Oct 15;272(2):299-305. doi: 10.1016/j.taap.2013.06.016. Epub 2013 Jun 26. Toxicol Appl Pharmacol. 2013. PMID: 23811326 Free PMC article.
-
Epigenetic inhibition of nuclear receptor small heterodimer partner is associated with and regulates hepatocellular carcinoma growth.Gastroenterology. 2008 Mar;134(3):793-802. doi: 10.1053/j.gastro.2008.01.006. Epub 2008 Jan 10. Gastroenterology. 2008. PMID: 18325392
-
Nuclear receptor SHP, a death receptor that targets mitochondria, induces apoptosis and inhibits tumor growth.Mol Cell Biol. 2010 Mar;30(6):1341-56. doi: 10.1128/MCB.01076-09. Epub 2010 Jan 11. Mol Cell Biol. 2010. PMID: 20065042 Free PMC article.
-
Structure and function of the atypical orphan nuclear receptor small heterodimer partner.Int Rev Cytol. 2007;261:117-58. doi: 10.1016/S0074-7696(07)61003-1. Int Rev Cytol. 2007. PMID: 17560281 Review.
Cited by
-
The long and the small collide: LncRNAs and small heterodimer partner (SHP) in liver disease.Mol Cell Endocrinol. 2021 May 15;528:111262. doi: 10.1016/j.mce.2021.111262. Epub 2021 Mar 26. Mol Cell Endocrinol. 2021. PMID: 33781837 Free PMC article. Review.
-
A pleiotropic role for the orphan nuclear receptor small heterodimer partner in lipid homeostasis and metabolic pathways.J Lipids. 2012;2012:304292. doi: 10.1155/2012/304292. Epub 2012 Apr 22. J Lipids. 2012. PMID: 22577560 Free PMC article.
-
Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice.Mol Endocrinol. 2012 Feb;26(2):272-80. doi: 10.1210/me.2011-1157. Epub 2012 Jan 19. Mol Endocrinol. 2012. PMID: 22261820 Free PMC article.
-
A feedback inhibition between miRNA-127 and TGFβ/c-Jun cascade in HCC cell migration via MMP13.PLoS One. 2013 Jun 7;8(6):e65256. doi: 10.1371/journal.pone.0065256. Print 2013. PLoS One. 2013. PMID: 23762330 Free PMC article.
-
Loss of the orphan nuclear receptor SHP is more pronounced in fibrolamellar carcinoma than in typical hepatocellular carcinoma.PLoS One. 2012;7(1):e30944. doi: 10.1371/journal.pone.0030944. Epub 2012 Jan 23. PLoS One. 2012. PMID: 22292081 Free PMC article.
References
-
- Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. - PubMed
-
- Harper JW, Elledge SJ. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–64. - PubMed
-
- Bates SL, Bonetta L, Macallan D, Parry D, Holder A, Dickson C, et al. CDK6 (PLSTIRE) and CDK4 (PSKJ3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene. 1994;9:71–79. - PubMed
-
- Mayol X, Garriga J, Grana X. Cell cycle-dependent phosphorylation of the retinoblastome-related protein p130. Oncogene. 1995;11:801–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
