Ionothermal synthesis of homochiral framework with acetate-pillared cobalt-camphorate architecture
- PMID: 18537237
- PMCID: PMC2766170
- DOI: 10.1021/ic8007798
Ionothermal synthesis of homochiral framework with acetate-pillared cobalt-camphorate architecture
Abstract
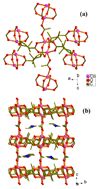
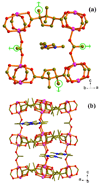
A new organically templated homochiral material (EMIm)[Co 2( d-cam) 2(ac)] ( 1; d-H 2cam = d-camphoric acid; ac = acetate; EMIm = 1-ethyl-3-methylimidazolium) has been ionothermally synthesized, and it features an unusual acetate-pillared cobalt-camphorate architecture encapsulating the cationic component of the ionic liquid.
Figures

Similar articles
-
Structure-directing effects of ionic liquids in the ionothermal synthesis of metal-organic frameworks.IUCrJ. 2017 Jun 30;4(Pt 4):380-392. doi: 10.1107/S2052252517008326. eCollection 2017 Jul 1. IUCrJ. 2017. PMID: 28875025 Free PMC article. Review.
-
Three-dimensional homochiral transition-metal camphorate architectures directed by a flexible auxiliary ligand.Inorg Chem. 2008 May 5;47(9):3495-7. doi: 10.1021/ic800195u. Epub 2008 Apr 8. Inorg Chem. 2008. PMID: 18393493
-
Structure-property relationship of homochiral and achiral supramolecular isomers obtained by one-pot synthesis.Chem Commun (Camb). 2012 Nov 11;48(87):10757-9. doi: 10.1039/c2cc36003a. Chem Commun (Camb). 2012. PMID: 23011555
-
Absolute helicity induction in three-dimensional homochiral frameworks.Chem Commun (Camb). 2009 Jan 8;(2):206-8. doi: 10.1039/b816742g. Epub 2008 Nov 13. Chem Commun (Camb). 2009. PMID: 19099070 Free PMC article.
-
The Relationship between the Structure and Properties of Amino Acid Ionic Liquids.Molecules. 2019 Sep 6;24(18):3252. doi: 10.3390/molecules24183252. Molecules. 2019. PMID: 31500119 Free PMC article. Review.
Cited by
-
Pseudohalogen Chemistry in Ionic Liquids with Non-innocent Cations and Anions.ChemistryOpen. 2021 Feb;10(2):62-71. doi: 10.1002/open.202000252. Epub 2020 Nov 10. ChemistryOpen. 2021. PMID: 33565728 Free PMC article. Review.
-
Structure-directing effects of ionic liquids in the ionothermal synthesis of metal-organic frameworks.IUCrJ. 2017 Jun 30;4(Pt 4):380-392. doi: 10.1107/S2052252517008326. eCollection 2017 Jul 1. IUCrJ. 2017. PMID: 28875025 Free PMC article. Review.
-
Ionothermal synthesis of open-framework metal phosphates with a Kagomé lattice network exhibiting canted anti-ferromagnetism†Electronic supplementary information (ESI) available: Cif files, atomic parameters, X-ray diffraction patterns, IR spectra, TG curves, and thermal ellipsoid plot and atomic label schemes of compound 1-4. See DOI: 10.1039/c4tc00290cClick here for additional data file.J Mater Chem C Mater. 2014 Sep 21;2(35):7417-7427. doi: 10.1039/c4tc00290c. Epub 2014 Aug 4. J Mater Chem C Mater. 2014. PMID: 25580250 Free PMC article.
-
Inorganic Synthesis Based on Reactions of Ionic Liquids and Deep Eutectic Solvents.Angew Chem Int Ed Engl. 2021 Oct 4;60(41):22148-22165. doi: 10.1002/anie.202104035. Epub 2021 Jul 1. Angew Chem Int Ed Engl. 2021. PMID: 34032351 Free PMC article. Review.
References
-
- Seo JS, Whang D, Lee H, Jun SI, Oh J, Jeon YJ, Kim K. Nature. 2000;404:982. - PubMed
- Vaidhyanathan R, Bradshaw D, Rebilly J-N, Barrio JP, Gould JA, Berry NG, Rosseinsky MJ. Angew. Chem. Int. Ed. 2006;45:6495. - PubMed
- Kepert CJ, Prior TJ, Rosseinsky MJ. J. Am. Chem. Soc. 2000;122:5158.
- Bradshaw D, Prior TJ, Cussen EJ, Claridge JB, Rosseinsky MJ. J. Am. Chem. Soc. 2004;126:6106. - PubMed
-
- Zhang J, Bu X. Angew. Chem. Int. Ed. 2007;46:6115. - PubMed
- Zhang J, Chen S, Valle H, Wong M, Austria C, Cruz M, Bu X. J. Am. Chem. Soc. 2007;129:14168. - PMC - PubMed
- Zhang J, Yao Y-G, Bu X. Chem. Mater. 2007;19:5083.
- Zhang J, Bu X. Chem. Commun. 2008:444. - PubMed
- Zhang J, Liu R, Feng P, Bu X. Angew. Chem. Int. Ed. 2007;46:8388. - PubMed
- Zhang J, Chew E, Chen S, Pham JTH, Bu X. Inorg. Chem. 2008;47:3495. - PubMed
-
- Attfield MP, Al-Ebini Y, Pritchard RG, Andrews EM, Charlesworth RJ, Hung W, Masheder BJ, Royal DS. Chem. Mater. 2007;19:316.
- Harvey G, Herve AC, Hailes HC, Attfield MP. Chem. Mater. 2004;16:3756.
- Harvey G, Teat SJ, Tang CC, Cranswick LM, Attfield MP. Inorg. Chem. 2003;42:2428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

