Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer
- PMID: 18538140
- PMCID: PMC2667819
- DOI: 10.1053/j.gastro.2008.04.022
Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer
Abstract
Background & aims: Toll-like receptor (TLR)-dependent signaling was proposed as immunotherapeutic targets against invading pathogens and tumorigenesis. Here, we investigated whether TLR5-dependent signaling modulates colonic tumor development in mouse xenograft model of human colon cancer.
Methods: The expression of myeloid differentiation factor 88 (MyD88) or TLR5 was stably knocked down in human colon cancer cells (DLD-1). Nude mice were subcutaneously implanted with MyD88-knocked down (KD), TLR5-KD, or control cells (n = 16) to examine the pathophysiology of tumor xenografts. Protein microarray assessed the differential expression of cytokines in these tumors. Leukocyte infiltration and tumor angiogenesis were assessed by immunohistochemistry with antibodies against neutrophil (Gr-1, 7/4) or macrophage-specific antigens (CD68, F4-80) and the vascular endothelial cell marker CD31, respectively. Tumor xenografts from DLD-1 cells were treated with flagellin (5.0 microg/kg, 1 injection/every 2 days for 3 weeks), and tumor regression and histopathology were examined.
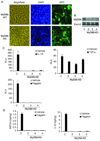
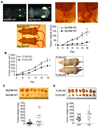
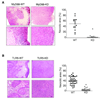
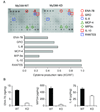
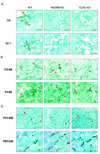
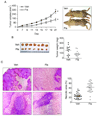
Results: Lack of MyD88 or TLR5 expression dramatically enhanced tumor growth and inhibited tumor necrosis in mouse xenografts of human colon cancer. In contrast, TLR5 activation by peritumoral flagellin treatment substantially increased tumor necrosis, leading to significant tumor regression. Tumors from MyD88-KD or TLR5-KD cells revealed the reduced production of neutrophil attracting chemokines (epithelial cell-derived neutrophil-activating peptide-78, macrophage-inflammatory protein alpha, and interleukin-8). Consequently, neutrophil infiltration was dramatically diminished in MyD88- or TLR5-KD xenografts, whereas tumor-associated macrophage infiltration or angiogenesis was not changed.
Conclusions: TLR5 engagement by flagellin mediates innate immunity and elicits potent antitumor activity, indicating that TLR5-dependent signaling could be a potential immunotherapeutic target to modulate colonic tumors.
Figures
Comment in
-
Guardians of the gut: newly appreciated role of epithelial toll-like receptors in protecting the intestine.Gastroenterology. 2008 Aug;135(2):351-4. doi: 10.1053/j.gastro.2008.06.064. Epub 2008 Jul 11. Gastroenterology. 2008. PMID: 18619968 No abstract available.
References
-
- Seliger B, Maeurer MJ, Ferrone S. TAP off--tumors on. Immunol Today. 1997;18:292–299. - PubMed
-
- Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. - PubMed
-
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. - PubMed
-
- Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. - PubMed
-
- Rhee SH, Hwang D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J Biol Chem. 2000;275:34035–34040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

