Specific contribution of lamin A and lamin C in the development of laminopathies
- PMID: 18538321
- PMCID: PMC3934841
- DOI: 10.1016/j.yexcr.2008.04.017
Specific contribution of lamin A and lamin C in the development of laminopathies
Abstract
Mutations in the lamin A/C gene are involved in multiple human disorders for which the pathophysiological mechanisms are partially understood. Conflicting results prevail regarding the organization of lamin A and C mutants within the nuclear envelope (NE) and on the interactions of each lamin to its counterpart. We over-expressed various lamin A and C mutants both independently and together in COS7 cells. When expressed alone, lamin A with cardiac/muscular disorder mutations forms abnormal aggregates inside the NE and not inside the nucleoplasm. Conversely, the equivalent lamin C organizes as intranucleoplasmic aggregates that never connect to the NE as opposed to wild type lamin C. Interestingly, the lamin C molecules present within these aggregates exhibit an abnormal increased mobility. When co-expressed, the complex formed by lamin A/C aggregates in the NE. Lamin A and C mutants for lipodystrophy behave similarly to the wild type. These findings reveal that lamins A and C may be differentially affected depending on the mutation. This results in multiple possible physiological consequences which likely contribute in the phenotypic variability of laminopathies. The inability of lamin C mutants to join the nuclear rim in the absence of lamin A is a potential pathophysiological mechanism for laminopathies.
Figures

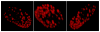


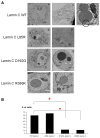
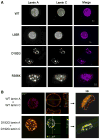



References
-
- Sylvius N, Tesson F. Lamin A/C and cardiac diseases. Curr Opin Cardiol. 2006;21:159–165. - PubMed
-
- Shumaker DK, Kuczmarski ER, Goldman RD. The nucleoskeleton: lamins and actin are major players in essential nuclear functions. Curr Opin Cell Biol. 2003;15:358–366. - PubMed
-
- Hutchison CJ. Lamins: building blocks or regulators of gene expression? Nat Rev, Mol Cell Biol. 2002;3:848–858. - PubMed
-
- Boguslavsky RL, Stewart CL, Worman HJ. Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2006;15:653–663. - PubMed
-
- Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

