Enhancement of immunostimulatory properties of exosomal vaccines by incorporation of fusion-competent G protein of vesicular stomatitis virus
- PMID: 18538453
- PMCID: PMC7115564
- DOI: 10.1016/j.vaccine.2008.04.069
Enhancement of immunostimulatory properties of exosomal vaccines by incorporation of fusion-competent G protein of vesicular stomatitis virus
Abstract
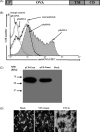
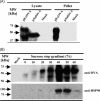
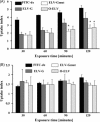
Exosomes have been proposed as candidates for therapeutic immunization. The present study demonstrates that incorporation of the G protein of vesicular stomatitis virus (VSV-G) into exosome-like vesicles (ELVs) enhances their uptake and induces the maturation of dendritic cells. Targeting of VSV-G and ovalbumin as a model antigen to the same ELVs increased the cross-presentation of ovalbumin via an endosomal acidification mechanism. Immunization of mice with VSV-G and ovalbumin containing ELVs led to an increased IgG2a antibody response, expansion of antigen-specific CD8 T cells, strong in vivo CTL responses, and protection from challenge with ovalbumin expressing tumor cells. Thus, incorporation of VSV-G and targeting of antigens to ELVs are attractive strategies to improve exosomal vaccines.
Figures

References
-
- Zitvogel L., Fernandez N., Lozier A., Wolfers J., Regnault A., Raposo G. Dendritic cells or their exosomes are effective biotherapies of cancer. Eur J Cancer. 1999;35(Suppl 3):36–38. - PubMed
-
- Andre F., Schartz N.E., Chaput N., Flament C., Raposo G., Amigorena S. Tumor-derived exosomes: a new source of tumor rejection antigens. Vaccine. 2002;20(Suppl 4):A28–A31. - PubMed
-
- Chaput N., Schartz N.E., Andre F., Taieb J., Novault S., Bonnaventure P. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172:2137–2146. - PubMed
-
- Taieb J., Chaput N., Zitvogel L. Dendritic cell-derived exosomes as cell-free peptide-based vaccines. Crit Rev Immunol. 2005;25:215–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

