Genetic causes of erythrocytosis and the oxygen-sensing pathway
- PMID: 18538455
- PMCID: PMC3400270
- DOI: 10.1016/j.blre.2008.04.003
Genetic causes of erythrocytosis and the oxygen-sensing pathway
Abstract
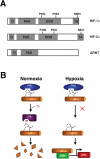
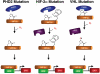
Idiopathic erythrocytosis is an uncommon disease, and is defined by an increase in red blood cell mass. The differential diagnosis of erythrocytosis is extensive, and can be divided into primary and secondary forms. Primary erythrocytoses are due to intrinsic defects in erythroid precursor cells and are characterized by low erythropoietin levels. Secondary erythrocytoses are extrinsic to erythroid progenitors and are characterized by either high or inappropriately normal erythropoietin levels. A distinct subset of secondary erythrocytoses are due to genetic mutations in key proteins of the oxygen-sensing pathway. These proteins constitute the core molecular machinery of oxygen-sensing with respect to red blood cell control. Apart from assigning physiologic roles for these proteins, studies of these rare mutations have (i) revealed the exquisite sensitivity of this pathway to genetic perturbations, (ii) highlighted important functional regions of the proteins, and (iii) provided a basis for potentially targeting this pathway for therapeutic benefit.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

