The RNA polymerase II trigger loop functions in substrate selection and is directly targeted by alpha-amanitin
- PMID: 18538653
- PMCID: PMC2475549
- DOI: 10.1016/j.molcel.2008.04.023
The RNA polymerase II trigger loop functions in substrate selection and is directly targeted by alpha-amanitin
Abstract
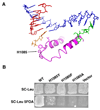
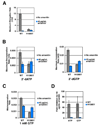
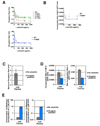
Structural, biochemical, and genetic studies have led to proposals that a mobile element of multisubunit RNA polymerases, the Trigger Loop (TL), plays a critical role in catalysis and can be targeted by antibiotic inhibitors. Here we present evidence that the Saccharomyces cerevisiae RNA Polymerase II (Pol II) TL participates in substrate selection. Amino acid substitutions within the Pol II TL preferentially alter substrate usage and enzyme fidelity, as does inhibition of transcription by alpha-amanitin. Finally, substitution of His1085 in the TL specifically renders Pol II highly resistant to alpha-amanitin, indicating a functional interaction between His1085 and alpha-amanitin that is supported by rerefinement of an alpha-amanitin-Pol II crystal structure. We propose that alpha-amanitin-inhibited Pol II elongation, which is slow and exhibits reduced substrate selectivity, results from direct alpha-amanitin interference with the TL.
Figures



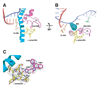
References
-
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58:1948–1954. - PubMed
-
- Bar-Nahum G, Epshtein V, Ruckenstein AE, Rafikov R, Mustaev A, Nudler E. A ratchet mechanism of transcription elongation and its control. Cell. 2005;120:183–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

