Potentiation of IL-19 expression in airway epithelia by IL-17A and IL-4/IL-13: important implications in asthma
- PMID: 18539194
- PMCID: PMC4631388
- DOI: 10.1016/j.jaci.2008.04.016
Potentiation of IL-19 expression in airway epithelia by IL-17A and IL-4/IL-13: important implications in asthma
Abstract
Background: IL-17A and IL-19 are highly expressed in chronic inflammatory diseases, such as psoriasis and asthma. IL-19 plays a significant role in the enhancement of T(H)2 cytokine secretion in allergic diseases, but its cellular source in asthmatic patients remains unknown.
Objective: Our aims were to determine whether the epithelium is a major source of airway mucosal IL-19 and to elucidate the mechanism of gene expression regulation.
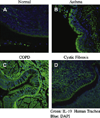
Methods: Immunofluorescent staining was used to determine IL-19 protein expression in tracheal tissue sections of various airway diseases. Well-differentiated primary human bronchial epithelial cultures and a corresponding cell line were used as in vitro models to study gene regulation.
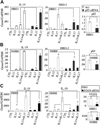
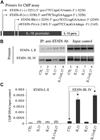
Results: We found significantly higher IL-19 expression in airway epithelia of asthmatic patients than in epithelia of patients with other diseases. Using a cytokine panel, we demonstrated the upregulation of IL-19 expression in cultures by two T(H)2 cytokines, IL-4 and IL-13, in addition to the previously found T(H)17 cytokine IL-17A. Moreover, cotreatment of IL-17A and IL-4/IL-13 synergistically upregulated IL-19 expression. Using siRNA and chemical inhibitor approaches, we demonstrated a transcriptional regulation of IL-19 by nuclear factor kappaB and signal transducer and activator of transcription (STAT) 6. The addition of IL-13 to IL-17A stimulation triggers a shift from nuclear factor kappaB-dependent transcriptional regulation to one that is STAT6 based. Using chromatin immunoprecipitation assays, we demonstrated the presence of STAT6-binding elements in the IL-19 promoter region.
Conclusion: We propose that an IL-17A- and IL-13-induced synergism in IL-19 stimulation in airway epithelia occurs through a STAT6-dependent pathway.
Conflict of interest statement
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
Figures



References
-
- Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, et al. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–450. - PubMed
-
- Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. - PubMed
-
- Conti P, Kempuraj D, Frydas S, Kandere K, Boucher W, Letourneau R, et al. IL-10 subfamily members: IL-19, IL-20, IL-22, IL-24 and IL-26. Immunol Lett. 2003;88:171–174. - PubMed
-
- Chang C, Magracheva E, Kozlov S, Fong S, Tobin G, Kotenko S, et al. Crystal structure of interleukin-19 defines a new subfamily of helical cytokines. J Biol Chem. 2003;278:3308–3313. - PubMed
-
- Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

