Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma
- PMID: 18539480
- PMCID: PMC2633865
- DOI: 10.1016/j.smim.2008.04.001
Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma
Abstract
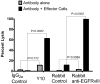
Conventional therapies for malignant gliomas (MGs) fail to target tumor cells exclusively, such that their efficacy is ultimately limited by non-specific toxicity. Immunologic targeting of tumor-specific gene mutations, however, may allow more precise eradication of neoplastic cells. The epidermal growth factor receptor variant III (EGFRvIII) is a consistent tumor-specific mutation that is widely expressed in MGs and other neoplasms. This mutation encodes a constitutively active tyrosine kinase that enhances tumorgenicity and migration and confers radiation and chemotherapeutic resistance. This in-frame deletion mutation splits a codon resulting in the creation of a novel glycine at the fusion junction between normally distant parts of the molecule and producing a sequence re-arrangement which creates a tumor-specific epitope for cellular or humoral immunotherapy in patients with MGs. We have previously shown that vaccination with a peptide that spans the EGFRvIII fusion junction is an efficacious immunotherapy in syngeneic murine models, but patients with MGs have a profound immunosuppression that may inhibit the ability of antigen presenting cells (APCs), even those generated ex vivo, to induce EGFRvIII-specific immune responses. In this report, we summarize our results in humans targeting this mutation in two consecutive and one multi-institutional Phase II immunotherapy trials. These trials demonstrated that vaccines targeting EGFRvIII are capable of inducing potent T- and B-cell immunity in these patients, and lead to an unexpectedly long survival time. Most importantly, vaccines targeting EGFRvIII were universally successful at eliminating tumor cells expressing the targeted antigen without any evidence of symptomatic collateral toxicity. These studies establish the tumor-specific EGFRvIII mutation as a novel target for humoral- and cell-mediated immunotherapy in a variety of cancers. The recurrence of EGFRvIII-negative tumors in our patients, however, highlights the need for targeting a broader repertoire of tumor-specific antigens.
Figures

References
-
- United States Cancer Statistics: National Program of Cancer Registries. 2003. Available from: http://apps.nccd.cdc.gov/uscs/
-
- CBTRUS. Statistical Report: Primary Brain Tumors in the United States, 1997–2001. Central Brain Tumor Registry of the United States, 2004; 2004.
-
- Kelly PJ. Stereotactic resection and its limitations in glial neoplasms. Stereotact Funct Neurosurg. 1992;59:84–91. - PubMed
-
- Walker MD, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–9. - PubMed
-
- Reardon DA, et al. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: phase II study results. J Clin Oncol. 2006;24:115–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

