Direct and potent regulation of gamma-secretase by its lipid microenvironment
- PMID: 18539594
- PMCID: PMC2504869
- DOI: 10.1074/jbc.M801925200
Direct and potent regulation of gamma-secretase by its lipid microenvironment
Abstract
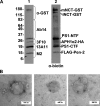
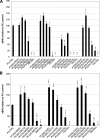
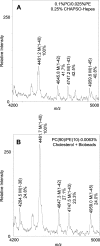
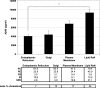
gamma-Secretase is an unusual and ubiquitous aspartyl protease with an intramembrane catalytic site that cleaves many type-I integral membrane proteins, most notably APP and Notch. Several reports suggest that cleavage of APP to produce the Abeta peptide is regulated in part by lipids. As gamma-secretase is a multipass protein complex with 19 transmembrane domains, it is likely that the local lipid composition of the membrane can regulate gamma-activity. To determine the direct contribution of the lipid microenvironment to gamma-secretase activity, we purified the human protease from overexpressing mammalian cells, reconstituted it in vesicles of varying lipid composition, and examined the effects of individual phospholipids, sphingolipids, cholesterol, and complex lipid mixtures on substrate cleavage. A conventional gamma-activity assay was modified to include a detergent-removal step to facilitate proteoliposome formation, and this increased baseline activity over 2-fold. Proteoliposomes containing sphingolipids significantly increased gamma-secretase activity over a phosphatidylcholine-only baseline, whereas the addition of phosphatidylinositol significantly decreased activity. Addition of soluble cholesterol in the presence of phospholipids and sphingolipids robustly increased the cleavage of APP- and Notch-like substrates in a dose-dependent manner. Reconstitution of gamma-secretase in complex lipid mixtures revealed that a lipid raft-like composition supported the highest level of activity compared with other membrane compositions. Taken together, these results demonstrate that membrane lipid composition is a direct and potent modulator of gamma-secretase and that cholesterol, in particular, plays a major regulatory role.
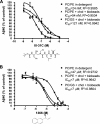
Figures


References
-
- Selkoe, D. J. (2001) Physiol. Rev. 81 741-766 - PubMed
-
- Edbauer, D., Winkler, E., Regula, J. T., Pesold, B., Steiner, H., and Haass, C. (2003) Nat. Cell Biol. 5 486-488 - PubMed
-
- Takasugi, N., Tomita, T., Hayashi, I., Tsuruoka, M., Niimura, M., Takahashi, Y., Thinakaran, G., and Iwatsubo, T. (2003) Nature 422 438-441 - PubMed
-
- Kopan, R., and Ilagan, M. X. (2004) Nat. Rev. Mol. Cell. Biol. 5 499-504 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

