Murine neonates develop vigorous in vivo cytotoxic and Th1/Th2 responses upon exposure to low doses of NIMA-like alloantigens
- PMID: 18539903
- PMCID: PMC2515119
- DOI: 10.1182/blood-2007-08-106500
Murine neonates develop vigorous in vivo cytotoxic and Th1/Th2 responses upon exposure to low doses of NIMA-like alloantigens
Abstract
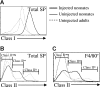
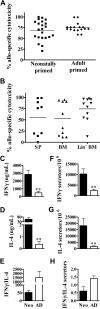
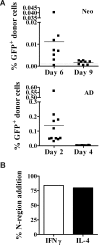
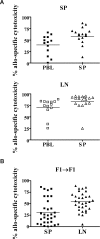
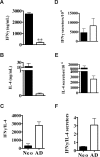
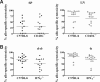
Early life exposure to noninherited maternal antigens (NIMAs) may occur via transplacental transfer and/or breast milk. There are indications that early life exposure to NIMAs may lead to lifelong tolerance. However, there is mounting evidence that exposure to NIMAs may also lead to immunologic priming. Understanding how these different responses arise could be critical in transplantation with donor cells expressing NIMAs. We recently reported that murine neonates that received a transplant of low doses of NIMA-like alloantigens develop vigorous memory cytotoxic responses, as assessed by in vitro assays. Here, we demonstrate that robust allospecific cytotoxicity is also manifest in vivo. Importantly, at low doses, NIMA-expressing cells induced the development of in vivo cytotoxicity during the neonatal period. NIMA-exposed neonates also developed vigorous primary and memory allospecific Th1/Th2 responses that exceeded the responses of adults. Overall, we conclude that exposure to low doses of NIMA-like alloantigens induces robust in vivo cytotoxic and Th1/Th2 responses in neonates. These findings suggest that early exposure to low levels of NIMA may lead to long-term immunologic priming of all arms of T-cell adaptive immunity, rather than tolerance.
Figures

Similar articles
-
The primary responses of murine neonatal lymph node CD4+ cells are Th2-skewed and are sufficient for the development of Th2-biased memory.Clin Dev Immunol. 2003 Mar;10(1):43-51. doi: 10.1080/10446670310001598474. Clin Dev Immunol. 2003. PMID: 14575157 Free PMC article.
-
The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates.J Immunol. 2001 Jan 15;166(2):918-25. doi: 10.4049/jimmunol.166.2.918. J Immunol. 2001. PMID: 11145668
-
[Neonatal tolerance to alloantigens].Med Sci (Paris). 2014 Feb;30(2):166-72. doi: 10.1051/medsci/20143002014. Epub 2014 Feb 24. Med Sci (Paris). 2014. PMID: 24572115 Review. French.
-
Induction of TH1 and TH2 immunity in neonatal mice.Science. 1996 Mar 22;271(5256):1728-30. doi: 10.1126/science.271.5256.1728. Science. 1996. PMID: 8596934
-
Tolerance to noninherited maternal antigens in mice and humans.Curr Opin Organ Transplant. 2009 Aug;14(4):439-47. doi: 10.1097/MOT.0b013e32832d6683. Curr Opin Organ Transplant. 2009. PMID: 19512930 Free PMC article. Review.
Cited by
-
The effect of maternal grafts in early acute cellular rejection after pediatric living-donor liver transplantation.Pediatr Surg Int. 2019 Jul;35(7):765-771. doi: 10.1007/s00383-019-04487-0. Epub 2019 May 20. Pediatr Surg Int. 2019. PMID: 31111216
-
Neonatal T Follicular Helper Cells Are Lodged in a Pre-T Follicular Helper Stage Favoring Innate Over Adaptive Germinal Center Responses.Front Immunol. 2019 Aug 13;10:1845. doi: 10.3389/fimmu.2019.01845. eCollection 2019. Front Immunol. 2019. PMID: 31456798 Free PMC article.
-
Naturally acquired microchimerism: implications for transplantation outcome and novel methodologies for detection.Chimerism. 2014;5(2):24-39. doi: 10.4161/chim.28908. Chimerism. 2014. PMID: 24762743 Free PMC article. Review.
-
Maternal-fetal cellular trafficking: clinical implications and consequences.Curr Opin Pediatr. 2014 Jun;26(3):377-82. doi: 10.1097/MOP.0000000000000087. Curr Opin Pediatr. 2014. PMID: 24759226 Free PMC article. Review.
-
Cell intrinsic characteristics of human cord blood naïve CD4T cells.Immunol Lett. 2018 Jan;193:51-57. doi: 10.1016/j.imlet.2017.11.011. Epub 2017 Nov 24. Immunol Lett. 2018. PMID: 29180044 Free PMC article.
References
-
- Zarou DM, Lichtman HC, Hellman LM. The transmission of chromium-51 tagged maternal erythrocytes from mother to fetus. Am J Obstet Gynecol. 1964;88:565–571. - PubMed
-
- Shimamura M, Ohta S, Suzuki R, Yamazaki K. Transmission of maternal blood cells to the fetus during pregnancy: detection in mouse neonatal spleen by immunofluorescence flow cytometry and polymerase chain reaction. Blood. 1994;83:926–930. - PubMed
-
- Piotrowski P, Croy BA. Maternal cells are widely distributed in murine fetuses in utero. Biol Reprod. 1996;54:1103–1110. - PubMed
-
- Lo YM, Lo ES, Watson N, et al. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood. 1996;88:4390–4395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

