Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis
- PMID: 18539952
- PMCID: PMC2574529
- DOI: 10.1165/rcmb.2007-0448OC
Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis
Abstract
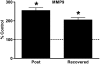
Lipopolysaccharide (LPS) is ubiquitous in the environment. Recent epidemiologic data suggest that occupational exposure to inhaled LPS can contribute to the progression of chronic obstructive pulmonary disease. To address the hypothesis that inhaled LPS can cause emphysema-like changes in mouse pulmonary parenchyma, we exposed C57BL/6 mice to aerosolized LPS daily for 4 weeks. By 3 days after the end of the 4-week exposure, LPS-exposed mice developed enlarged airspaces that persisted in the 4-week recovered mice. These architectural alterations in the lung are associated with enhanced type I, III, and IV procollagen mRNA as well as elevated levels of matrix metalloproteinase (MMP)-9 mRNA, all of which have been previously associated with human emphysema. Interestingly, MMP-9-deficient mice were not protected from the development of LPS-induced emphysema. However, we demonstrate that LPS-induced airspace enlargement was associated with apoptosis within the lung parenchyma, as shown by prominent TUNEL staining and elevated cleaved caspase 3 immunoreactivity. Antineutrophil antiserum-treated mice were partially protected from the lung destruction caused by chronic inhalation of LPS. Taken together, these findings demonstrate that inhaled LPS can cause neutrophil-dependent emphysematous changes in lung architecture that are associated with apoptosis and that these changes may be occurring through mechanisms different than those induced by cigarette smoke.
Figures





References
-
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet 1997;349:1498–1504. - PubMed
-
- Khan AJ, Nanchal R. Cotton dust lung diseases. Curr Opin Pulm Med 2007;13:137–141. - PubMed
-
- Castellan RM, Olenchock SA, Kinsley KB, Hankinson JL. Inhaled endotoxin and decreased spirometric values: an exposure-response relation for cotton dust. N Engl J Med 1987;317:605–610. - PubMed
-
- Wang XR, Zhang HX, Sun BX, Dai HL, Hang JQ, Eisen EA, Wegman DH, Olenchock SA, Christiani DC. A 20-year follow-up study on chronic respiratory effects of exposure to cotton dust. Eur Respir J 2005;26:881–886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

