Liver cancer stem cells
- PMID: 18539957
- PMCID: PMC2515096
- DOI: 10.1200/JCO.2007.15.5945
Liver cancer stem cells
Erratum in
- J Clin Oncol. 2008 Aug 1;26(22): 3819
Abstract
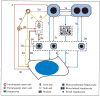
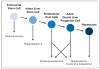
In an effort to review the evidence that liver cancer stem cells exist, two fundamental questions must be addressed. First, do hepatocellular carcinomas (HCC) arise from liver stem cells? Second, do HCCs contain cells that possess properties of cancer stem cells? For many years the finding of preneoplastic nodules in the liver during experimental induction of HCCs by chemicals was interpreted to support the hypothesis that HCC arose by dedifferentiation of mature liver cells. More recently, recognition of the role of small oval cells in the carcinogenic process led to a new hypothesis that HCC arises by maturation arrest of liver stem cells. Analysis of the cells in HCC supports the presence of cells with stem-cell properties (ie, immortality, transplantability, and resistance to therapy). However, definitive markers for these putative cancer stem cells have not yet been found and a liver cancer stem cell has not been isolated.
Figures

References
-
- Sell S, Pierce GB. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab Invest. 1994;70:6–22. - PubMed
-
- Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncology Hematology. 2004;52:1–28. - PubMed
-
- Sell S, Leffert HL. An evaluation of cellular lineages in the pathogenesis of experimental hepatocellular carcinoma. Hepatology. 1982;2:77–86. - PubMed
-
- Sell S. Cellular origin of hepatocellular carcinoma. Cell Develop Biology. 2002;13:419–424. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

