Mechanostimulation of Medicago truncatula leads to enhanced levels of jasmonic acid
- PMID: 18540020
- PMCID: PMC2486479
- DOI: 10.1093/jxb/ern145
Mechanostimulation of Medicago truncatula leads to enhanced levels of jasmonic acid
Abstract
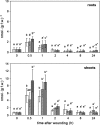
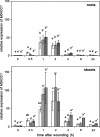
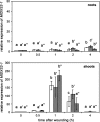
Wounding of plants leads to endogenous rise of jasmonic acid (JA) accompanied with the expression of a distinct set of genes. Among them are those coding for the allene oxide cyclase (AOC) that catalyses a regulatory step in JA biosynthesis, and for 1-deoxy-D-xylulose 5-phosphate synthase 2 (DXS2), an enzyme involved in isoprenoid biosynthesis. To address the question how roots and shoots of Medicago truncatula respond to mechanostimulation and wounding, M. truncatula plants were analysed in respect to JA levels as well as MtAOC1 and MtDXS2-1 transcript accumulation. Harvest-caused mechanostimulation resulted in a strong, but transient increase in JA level in roots and shoots followed by a transient increase in MtAOC1 transcript accumulation. Additional wounding of either shoots or roots led to further increased JA and MtAOC1 transcript levels in shoots, but not in roots. In situ hybridization revealed a cell-specific transcript accumulation of MtAOC1 after mechanostimulation in companion cells of the vascular tissue of the stem. AOC protein, however, was found to occur constitutively in vascular bundles. Further, transcript accumulation of MtDXS2-1 was similar to that of MtAOC1 in shoots, but its transcript levels were not enhanced in roots. Repeated touching of shoots increased MtAOC1 transcript levels and led to significantly shorter shoots and increased biomass. In conclusion, M. truncatula plants respond very sensitively to mechanostimulation with enhanced JA levels and altered transcript accumulation, which might contribute to the altered phenotype after repeated touching of plants.
Keywords: 1-deoxy-D-xylulose 5-phosphate synthase 2; Allene oxide cyclase; Medicago truncatula; cell specific expression; jasmonic acid; mechanostimulation; wounding.
Figures



References
-
- Afitlhile MM, Fukushige H, Nishimura M, Hildebrand DF. A defect in glyoxysomal fatty acid β-oxidation reduces jasmonic acid accumulation in Arabidopsis. Plant Physiology and Biochemistry. 2005;43:603–609. - PubMed
-
- Arimura G-i, Kost C, Boland W. Herbivore-induced, indirect plant defences. Biochimica et Biophysica Acta – Molecular and Cell Biology of Lipids. 2005;1734:91–111. - PubMed

