Phase 2, single-arm trial to evaluate the effectiveness of darbepoetin alfa for correcting anaemia in patients with myelodysplastic syndromes
- PMID: 18540943
- PMCID: PMC2654479
- DOI: 10.1111/j.1365-2141.2008.07181.x
Phase 2, single-arm trial to evaluate the effectiveness of darbepoetin alfa for correcting anaemia in patients with myelodysplastic syndromes
Abstract
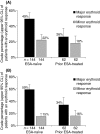
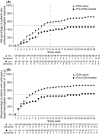
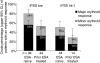
Patients with myelodysplastic syndromes (MDS) often develop anaemia resulting in frequent transfusions and fatigue. Darbepoetin alfa is an erythropoiesis-stimulating agent (ESA) approved for treating chemotherapy-induced anaemia. This single-arm, phase 2 study examined the efficacy of darbepoetin alfa 500 microg every 3 weeks (Q3W) for treating anaemia in low-risk MDS patients (after 6 weeks, poor responders received darbepoetin alfa 500 microg every 2 weeks). The primary end-point was the incidence of erythroid responses (International Working Group criteria) after 13 weeks of therapy. Secondary end-points included the incidence of erythroid responses at weeks 28 and 55, [or weeks 27 and 53 for dose escalations to every two weeks (Q2W)], and safety parameters. Analyses were stratified by the patient's previous ESA therapy status [ESA-naïve (n = 144) vs. prior ESA-treated (n = 62)]. After 13 weeks of therapy, 49% of ESA-naïve patients and 26% of prior ESA-treated patients achieved a major erythroid response. After 53/55 weeks, 59% of ESA-naïve patients and 34% of prior ESA-treated patients achieved a major erythroid response; 82% of ESA-naïve patients and 55% of prior ESA-treated patients achieved target haemoglobin of 110 g/l. Thromboembolic or related adverse events occurred in 2% of patients; no pulmonary embolisms were reported. In conclusion, darbepoetin alfa, 500 microg Q3W appeared well tolerated and increased haemoglobin levels in low-risk MDS patients.
Trial registration: ClinicalTrials.gov NCT00095264.
Figures



References
-
- Alessandrino EP, Amadori S, Barosi G, Cazzola M, Grossi A, Liberato LN, Locatelli F, Marchetti M, Morra E, Rebulla P, Visani G, Tura S. Evidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of Hematology. Haematologica. 2002;87:1286–1306. - PubMed
-
- Allampallam K, Shetty V, Mundle S, Dutt D, Kravitz H, Reddy PL, Alvi S, Galili N, Saberwal GS, Anthwal S, Shaikh MW, York A, Raza A. Biological significance of proliferation, apoptosis, cytokines, and monocyte/macrophage cells in bone marrow biopsies of 145 patients with myelodysplastic syndrome. International Journal of Hematology. 2002;75:289–297. - PubMed
-
- Balducci L. Transfusion independence in patients with myelodysplastic syndromes: impact on outcomes and quality of life. Cancer. 2006;106:2087–2094. - PubMed
-
- Balleari E, Rossi E, Clavio M, Congiu A, Gobbi M, Grosso M, Secondo V, Spriano M, Timitilli S, Ghio R. Erythropoietin plus granulocyte colony-stimulating factor is better than erythropoietin alone to treat anemia in low-risk myelodysplastic syndromes: results from a randomized single-centre study. Annals of Hematology. 2006;85:174–180. - PubMed
-
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposals for the classification of the myelodysplastic syndromes. British Journal of Haematology. 1982;51:189–199. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

