Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice
- PMID: 18541363
- PMCID: PMC3413727
- DOI: 10.1016/j.canlet.2008.04.026
Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice
Abstract
Plasmonic photothermal therapy (PPTT) is a minimally-invasive oncological treatment strategy in which photon energy is selectively administered and converted into heat sufficient to induce cellular hyperthermia. The present work demonstrates the feasibility of in vivo PPTT treatment of deep-tissue malignancies using easily-prepared plasmonic gold nanorods and a small, portable, inexpensive near-infrared (NIR) laser. Dramatic size decreases in squamous cell carcinoma xenografts were observed for direct (P<0.0001) and intravenous (P<0.0008) administration of pegylated gold nanorods in nu/nu mice. Inhibition of average tumor growth for both delivery methods was observed over a 13-day period, with resorption of >57% of the directly-injected tumors and 25% of the intravenously-treated tumors.
Figures

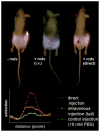
 ), intravenous (
), intravenous (
 ), and direct (
), and direct (
 ) administration of pegylated gold nanorods. Control mice were interstitially injected with 15 μL 10 mM PBS alone, while directly administered mice received interstitial injections of 15 μL pegylated gold nanorods (ODλ=800=40, 2 min accumulation), and intravenously administered mice recieved 100 μL pegylated gold nanorod (ODλ=800=120, 24 hr accumulation) injections.
) administration of pegylated gold nanorods. Control mice were interstitially injected with 15 μL 10 mM PBS alone, while directly administered mice received interstitial injections of 15 μL pegylated gold nanorods (ODλ=800=40, 2 min accumulation), and intravenously administered mice recieved 100 μL pegylated gold nanorod (ODλ=800=120, 24 hr accumulation) injections.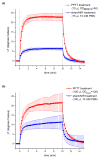
 ) and sham/NIR (
) and sham/NIR (
 ) treatments using pegylated gold nanorods. Direct PPTT treatments were performed by administration of 15 μL pegylated gold nanorods (ODλ=800=40, 2 min accumulation) followed by 10 min of 0.9–1.1 W/cm2 NIR laser exposure. Intravenous PPTT treatments were performed by administration of 100 μL pegylated gold nanorods (ODλ=800=120, 24 hr accumulation) followed by 10 min of 1.7–1.9 W/cm2 NIR laser exposure. Sham/NIR treatments were performed by administration of 15 μL 10 mM PBS and NIR laser exposure of comperable time and power density. Errors reported as standard deviation.
) treatments using pegylated gold nanorods. Direct PPTT treatments were performed by administration of 15 μL pegylated gold nanorods (ODλ=800=40, 2 min accumulation) followed by 10 min of 0.9–1.1 W/cm2 NIR laser exposure. Intravenous PPTT treatments were performed by administration of 100 μL pegylated gold nanorods (ODλ=800=120, 24 hr accumulation) followed by 10 min of 1.7–1.9 W/cm2 NIR laser exposure. Sham/NIR treatments were performed by administration of 15 μL 10 mM PBS and NIR laser exposure of comperable time and power density. Errors reported as standard deviation.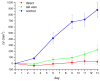
 ), intravenous (
), intravenous (
 ), and direct (
), and direct (
 ) injection of pegylated gold nanorods. Errors for control (n=10), direct injection (n=8), and intravenous injection (n=7) groups reported as standard error of the means. Control mice were treated by interstitial injection of 15 μL 10 mM PBS alone, while intravenous PPTT treatments were performed by administration of 100 μL pegylated gold nanorods (ODλ=800=120, 24 hr accumulation) followed by 10 min of 1.7–1.9 W/cm2 NIR laser exposure. Direct PPTT treatments were performed by administration of 15 μL pegylated gold nanorods (ODλ=800=40, 2 min accumulation) followed by 10 min of 0.9–1.1 W/cm2 NIR laser exposure.
) injection of pegylated gold nanorods. Errors for control (n=10), direct injection (n=8), and intravenous injection (n=7) groups reported as standard error of the means. Control mice were treated by interstitial injection of 15 μL 10 mM PBS alone, while intravenous PPTT treatments were performed by administration of 100 μL pegylated gold nanorods (ODλ=800=120, 24 hr accumulation) followed by 10 min of 1.7–1.9 W/cm2 NIR laser exposure. Direct PPTT treatments were performed by administration of 15 μL pegylated gold nanorods (ODλ=800=40, 2 min accumulation) followed by 10 min of 0.9–1.1 W/cm2 NIR laser exposure.References
-
- Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. - PubMed
-
- Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. - PubMed
-
- Katz E, Willner I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew Chem Int Edit. 2004;43:6042–6108. - PubMed
-
- Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol Rev. 2001;53:283–318. - PubMed
-
- Niemeyer CM. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew Chem-Int Edit. 2001;40:4128–4158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

