ERCC1-XPF endonuclease facilitates DNA double-strand break repair
- PMID: 18541667
- PMCID: PMC2519706
- DOI: 10.1128/MCB.00293-08
ERCC1-XPF endonuclease facilitates DNA double-strand break repair
Abstract
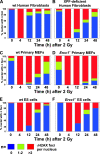
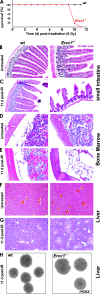
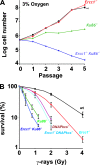
ERCC1-XPF endonuclease is required for nucleotide excision repair (NER) of helix-distorting DNA lesions. However, mutations in ERCC1 or XPF in humans or mice cause a more severe phenotype than absence of NER, prompting a search for novel repair activities of the nuclease. In Saccharomyces cerevisiae, orthologs of ERCC1-XPF (Rad10-Rad1) participate in the repair of double-strand breaks (DSBs). Rad10-Rad1 contributes to two error-prone DSB repair pathways: microhomology-mediated end joining (a Ku86-independent mechanism) and single-strand annealing. To determine if ERCC1-XPF participates in DSB repair in mammals, mutant cells and mice were screened for sensitivity to gamma irradiation. ERCC1-XPF-deficient fibroblasts were hypersensitive to gamma irradiation, and gammaH2AX foci, a marker of DSBs, persisted in irradiated mutant cells, consistent with a defect in DSB repair. Mutant mice were also hypersensitive to irradiation, establishing an essential role for ERCC1-XPF in protecting against DSBs in vivo. Mice defective in both ERCC1-XPF and Ku86 were not viable. However, Ercc1(-/-) Ku86(-/-) fibroblasts were hypersensitive to gamma irradiation compared to single mutants and accumulated significantly greater chromosomal aberrations. Finally, in vitro repair of DSBs with 3' overhangs led to large deletions in the absence of ERCC1-XPF. These data support the conclusion that, as in yeast, ERCC1-XPF facilitates DSB repair via an end-joining mechanism that is Ku86 independent.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
