Preclinical evaluation of 1H-benzylindole derivatives as novel human immunodeficiency virus integrase strand transfer inhibitors
- PMID: 18541726
- PMCID: PMC2493095
- DOI: 10.1128/AAC.00210-08
Preclinical evaluation of 1H-benzylindole derivatives as novel human immunodeficiency virus integrase strand transfer inhibitors
Abstract
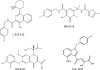
We have identified 1H-benzylindole analogues as a novel series of human immunodeficiency virus (HIV) integrase inhibitors with antiretroviral activities against different strains of HIV type 1 (HIV-1), HIV-2, and simian immunodeficiency virus strain MAC(251) [SIV(MAC(251))]. Molecular modeling and structure-activity relationship-based optimization resulted in the identification of CHI/1043 as the most potent congener. CHI/1043 inhibited the replication of HIV-1(III(B)) in MT-4 cells at a 50% effective concentration (EC(50)) of 0.60 microM, 70-fold below its cytotoxic concentration. Equal activities against HIV-1(NL4.3), HIV-2(ROD), HIV-2(EHO), and SIV(MAC(251)) were observed. CHI/1043 was equally active against virus strains resistant against inhibitors of reverse transcriptase or protease. Replication of both X4 and R5 strains in peripheral blood mononuclear cells was sensitive to the inhibitory effect of CHI/1043 (EC(50), 0.30 to 0.38 microM). CHI/1043 inhibited integrase strand transfer activity in oligonucleotide-based enzymatic assays at low micromolar concentrations. Time-of-addition experiments confirmed CHI/1043 to interfere with the viral replication cycle at the time of retroviral integration. Quantitative Alu PCR corroborated that the anti-HIV activity is based upon the inhibition of proviral DNA integration. An HIV-1 strain selected for 70 passages in the presence of CHI/1043 was evaluated genotypically and phenotypically. The mutations T66I and Q146K were present in integrase. Cross-resistance to other integrase strand transfer inhibitors, such as L-708,906, the naphthyridine analogue L-870,810, and the clinical drugs GS/9137 and MK-0518, was observed. In adsorption, distribution, metabolism, excretion, and toxicity studies, antiviral activity was strongly reduced by protein binding, and metabolization in human liver microsomes was observed. Transport studies with Caco cells suggest a low oral bioavailability.
Figures


Similar articles
-
New class of HIV integrase inhibitors that block viral replication in cell culture.Curr Biol. 2002 Jul 23;12(14):1169-77. doi: 10.1016/s0960-9822(02)00952-1. Curr Biol. 2002. PMID: 12176326
-
Development of resistance against diketo derivatives of human immunodeficiency virus type 1 by progressive accumulation of integrase mutations.J Virol. 2003 Nov;77(21):11459-70. doi: 10.1128/jvi.77.21.11459-11470.2003. J Virol. 2003. PMID: 14557631 Free PMC article.
-
HIV-1 integrase strand-transfer inhibitors: design, synthesis and molecular modeling investigation.Eur J Med Chem. 2011 Feb;46(2):756-64. doi: 10.1016/j.ejmech.2010.12.012. Epub 2010 Dec 21. Eur J Med Chem. 2011. PMID: 21227550
-
Advances in rationally designed dual inhibitors of HIV-1 reverse transcriptase and integrase.Bioorg Med Chem. 2016 Nov 1;24(21):5007-5016. doi: 10.1016/j.bmc.2016.09.025. Epub 2016 Sep 12. Bioorg Med Chem. 2016. PMID: 27658796 Review.
-
Targeting Integrase Enzyme: A Therapeutic Approach to Combat HIV Resistance.Mini Rev Med Chem. 2020;20(3):219-238. doi: 10.2174/1389557519666191015124932. Mini Rev Med Chem. 2020. PMID: 31613727 Review.
Cited by
-
Antiviral mode of action of bovine dialyzable leukocyte extract against human immunodeficiency virus type 1 infection.BMC Res Notes. 2011 Nov 1;4:474. doi: 10.1186/1756-0500-4-474. BMC Res Notes. 2011. PMID: 22044844 Free PMC article.
-
Response of a simian immunodeficiency virus (SIVmac251) to raltegravir: a basis for a new treatment for simian AIDS and an animal model for studying lentiviral persistence during antiretroviral therapy.Retrovirology. 2010 Mar 16;7:21. doi: 10.1186/1742-4690-7-21. Retrovirology. 2010. PMID: 20233398 Free PMC article.
-
Microwave assisted organic synthesis (MAOS) of small molecules as potential HIV-1 integrase inhibitors.Molecules. 2011 Aug 11;16(8):6858-70. doi: 10.3390/molecules16086858. Molecules. 2011. PMID: 21836543 Free PMC article.
-
Human immunodeficiency virus: 25 years of diagnostic and therapeutic strategies and their impact on hepatitis B and C virus.Med Microbiol Immunol. 2009 Aug;198(3):147-55. doi: 10.1007/s00430-009-0117-6. Epub 2009 Jun 4. Med Microbiol Immunol. 2009. PMID: 19495792 Free PMC article. Review.
-
Identification and characterization of persistent intracellular human immunodeficiency virus type 1 integrase strand transfer inhibitor activity.Antimicrob Agents Chemother. 2011 Jan;55(1):42-9. doi: 10.1128/AAC.01064-10. Epub 2010 Nov 8. Antimicrob Agents Chemother. 2011. PMID: 21060108 Free PMC article.
References
-
- Artursson, P., and J. Karlsson. 1991. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 175:880-885. - PubMed
-
- Barreca, M. L., S. Ferro, A. Rao, L. De Luca, M. Zappala, A. M. Monforte, Z. Debyser, M. Witvrouw, and A. Chimirri. 2005. Pharmacophore-based design of HIV-1 integrase strand-transfer inhibitors. J. Med. Chem. 48:7084-7088. - PubMed
-
- Barré-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vezinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868-871. - PubMed
-
- Condra, J. H., W. A. Schleif, O. M. Blahy, L. J. Gabryelski, D. J. Graham, J. C. Quintero, A. Rhodes, H. L. Robbins, E. Roth, M. Shivaprakash, et al. 1995. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature 374:569-571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information

