Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala
- PMID: 18541912
- PMCID: PMC2448850
- DOI: 10.1073/pnas.0802302105
Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala
Abstract
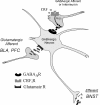
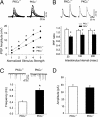
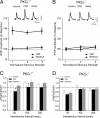
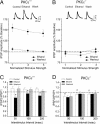
In the central amygdala (CeA), ethanol acts via corticotrophin-releasing factor (CRF) type 1 receptors to enhance GABA release. Amygdala CRF mediates anxiety associated with stress and drug dependence, and it regulates ethanol intake. Because mutant mice that lack PKCepsilon exhibit reduced anxiety-like behavior and alcohol consumption, we investigated whether PKCepsilon lies downstream of CRF(1) receptors in the CeA. Compared with PKCepsilon(+/+) CeA neurons, PKCepsilon(-/-) neurons showed increased GABAergic tone due to enhanced GABA release. CRF and ethanol stimulated GABA release in the PKCepsilon(+/+) CeA, but not in the PKCepsilon(-/-) CeA. A PKCepsilon-specific inhibitor blocked both CRF- and ethanol-induced GABA release in the PKCepsilon(+/+) CeA, confirming findings in the PKCepsilon(-/-) CeA. These results identify a PKCepsilon signaling pathway in the CeA that is activated by CRF(1) receptor stimulation, mediates GABA release at nerve terminals, and regulates anxiety and alcohol consumption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. - PubMed
-
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. - PubMed
-
- Campbell BM, Morrison JL, Walker EL, Merchant KM. Differential regulation of behavioral, genomic, and neuroendocrine responses by CRF infusions in rats. Pharmacol Biochem Behav. 2004;77:447–455. - PubMed
-
- Nishikawa H, Hata T, Itoh E, Funakami Y. A role for corticotropin-releasing factor in repeated cold stress-induced anxiety-like behavior during forced swimming and elevated plus-maze tests in mice. Biol Pharm Bull. 2004;27:352–356. - PubMed
-
- Smith GW, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AA013588/AA/NIAAA NIH HHS/United States
- AA015566/AA/NIAAA NIH HHS/United States
- AA013517/AA/NIAAA NIH HHS/United States
- R37 AA013588/AA/NIAAA NIH HHS/United States
- U01 AA013517/AA/NIAAA NIH HHS/United States
- R01 DA003665/DA/NIDA NIH HHS/United States
- DA03665/DA/NIDA NIH HHS/United States
- R01 AA015566/AA/NIAAA NIH HHS/United States
- U24 AA013517/AA/NIAAA NIH HHS/United States
- P50 AA006420/AA/NIAAA NIH HHS/United States
- AA06420/AA/NIAAA NIH HHS/United States
- AA10994/AA/NIAAA NIH HHS/United States
- AA013588/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

