Cisplatin and vinorelbine first-line chemotherapy in non-resectable malignant pleural mesothelioma
- PMID: 18542078
- PMCID: PMC2453034
- DOI: 10.1038/sj.bjc.6604421
Cisplatin and vinorelbine first-line chemotherapy in non-resectable malignant pleural mesothelioma
Abstract
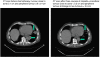
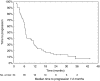
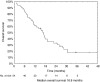
The aim was to evaluate the activity of cisplatin and vinorelbine in previously untreated, inoperable patients having histologically verified malignant pleural mesothelioma (MPM), normal organ function, and performance status 0-2. Treatment was vinorelbine 25 mg m(-2) i.v. weekly and cisplatin 100 mg m(-2) i.v. every 4 weeks with hydration and standard prophylactic antiemetic treatment. Patients gave written informed consent. Characteristics of 54 consecutive patients were: males 85%, epithelial subtype 74%, IMIG stages III and IV 35 and 46%, performance status 0, 1, and 2, 26, 69, and 6%, and median age 63 years (31-78 years). CTC grade 3 or 4 toxicity occurred with respect to leukocytopenia (48% of patients, grade 4 in 13%), nausea (13%), neurotoxicity (11%), nephrotoxicity (4%), and other toxicities (9%). There were no toxic deaths. The median number of cycles was four. The fraction of patients alive at 1-, 2-, and 3-years were 61, 31, and 4%, respectively, and median survival and median time to progression were 16.8 months (0.5 to 46.4 +months) and 7.2 months (1.6 to 40.6 + months). There were two CRs and 14 PRs (response rate 29.6%). Cisplatin and intravenous vinorelbine is a highly active regimen in MPM with a response rate and survival comparable to the most active regimens so far reported.
Figures

References
-
- Andreopoulou E, Ross PJ, O'Brien MER, Ford HER, Priest K, Eisen T, Norton A, Aschley S, Smith IE (2004) The palliative benefits of MVP (mitomycin C, vinblastine and cisplatin) chemotherapy in patients with malignant mesothelioma. Ann of Oncol 15: 1406–1412 - PubMed
-
- Berghmans T, Lafitte JJ, Paesmans M, Stach B, Berchier MC, Wackenier P, Lecomte J, Collon T, Mommen P, Sculier JP (2005) A phase II study evaluating the cisplatin and epirubicin combination in patients with unresectable malignant pleural mesothelioma. Cancer 50: 75–82. DOI 10.1016/j.lungcan.2005.05.007 - PubMed
-
- Berghmans T, Paesmans M, Lalami Y, Louviaux I, Luce S, Mascaux C, Meert AP, Schulier JP (2002) Activity of chemotherapy and immunotherapy on malignant mesothelioma: a systematic review of the literature with metaanalysis. Lung cancer 38: 111–121 - PubMed
-
- Bottomley A, Gaafa R, Manegold C, Burgers S, Coens C, Legrand C, Vincent M, Giaccone G, Van Meerbeeck J (2006) Short-term treatment related symptoms and quality of life: Results from an international randomized phase III study of Cisplatin with or without raltitrexed in patients with malignant pleural Mesothelioma: An EORTC lung-cancer group and National Cancer Institute, Canada, intergroup study. J Clin Oncol 24: 1435–1442 - PubMed
-
- Boyer MJ, Jassem J, Liepa AM (2003) Symptom and quality of life advantages for pemetrexed+Cisplatin versus Cisplatin alone in treatment of malignant pleural Mesothelioma. Lung Cancer 41(Suppl 2): S19
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

